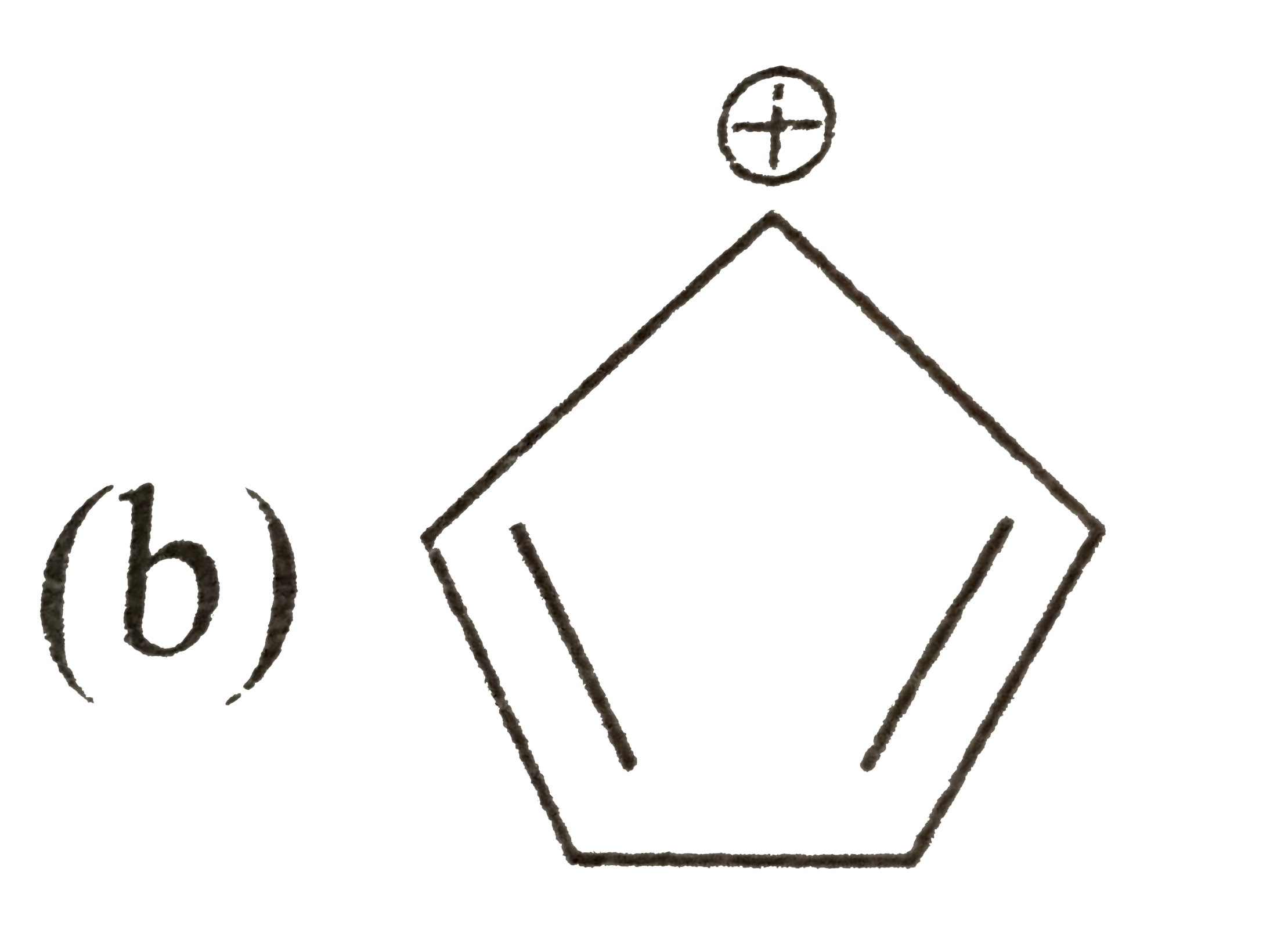
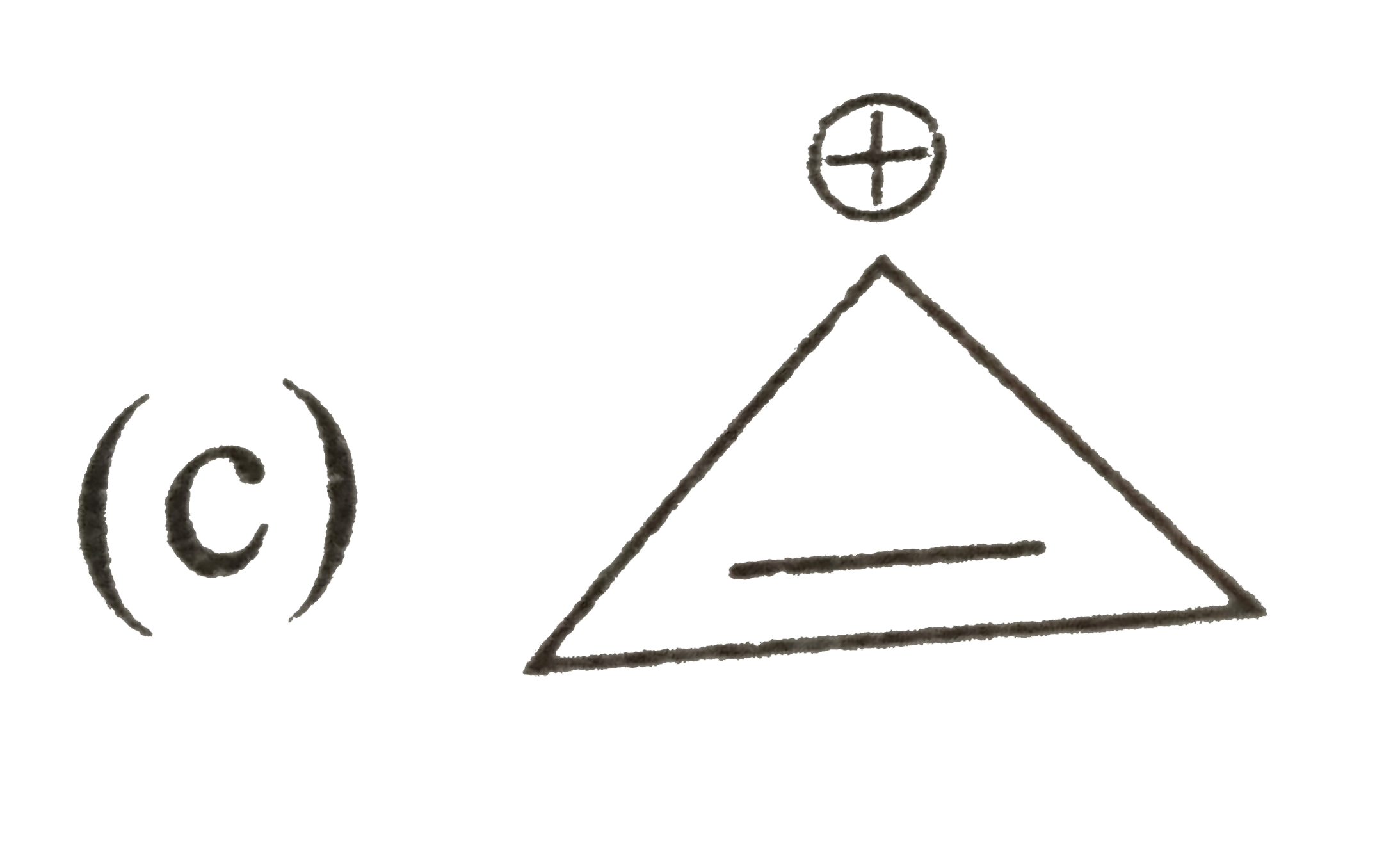
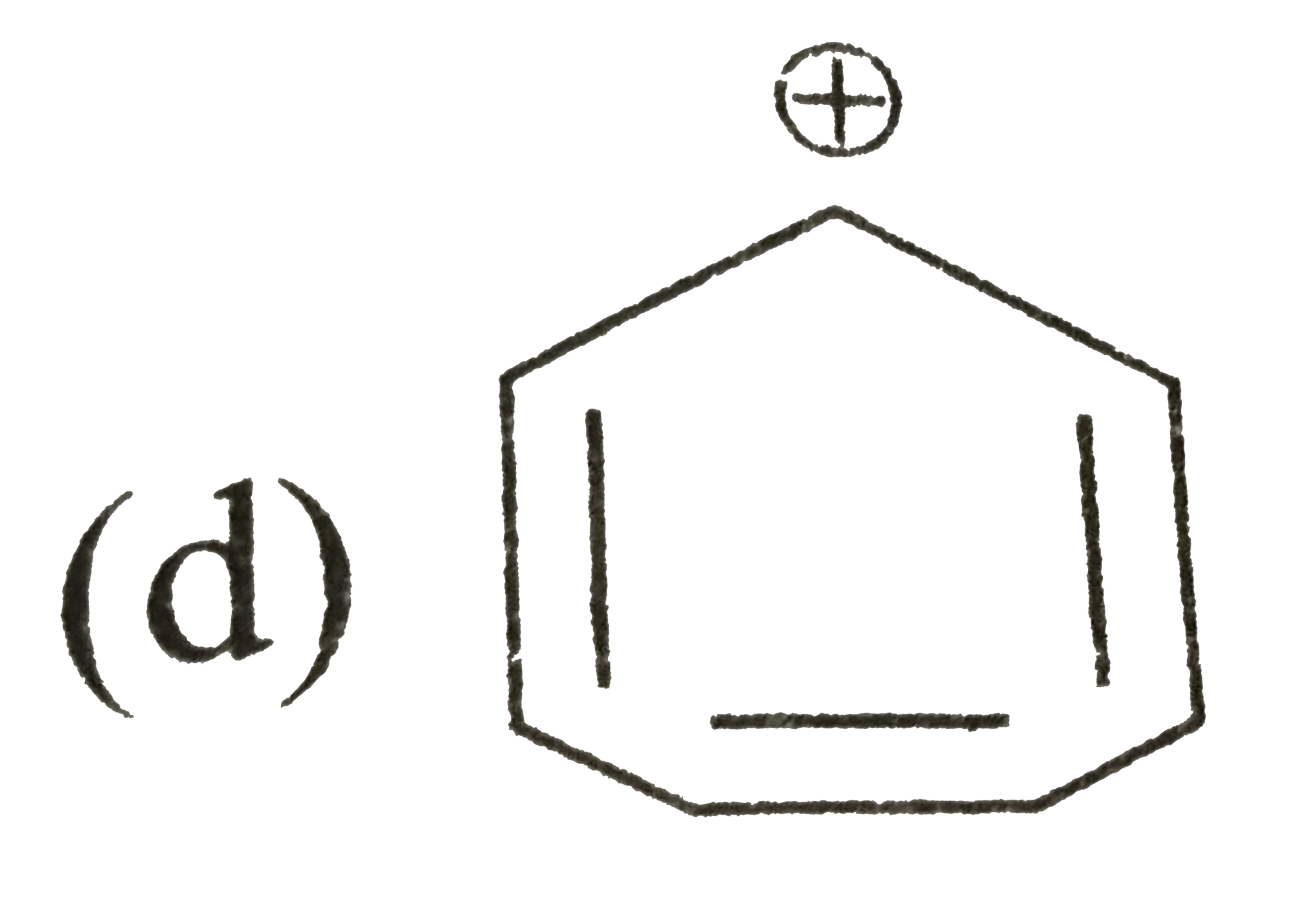
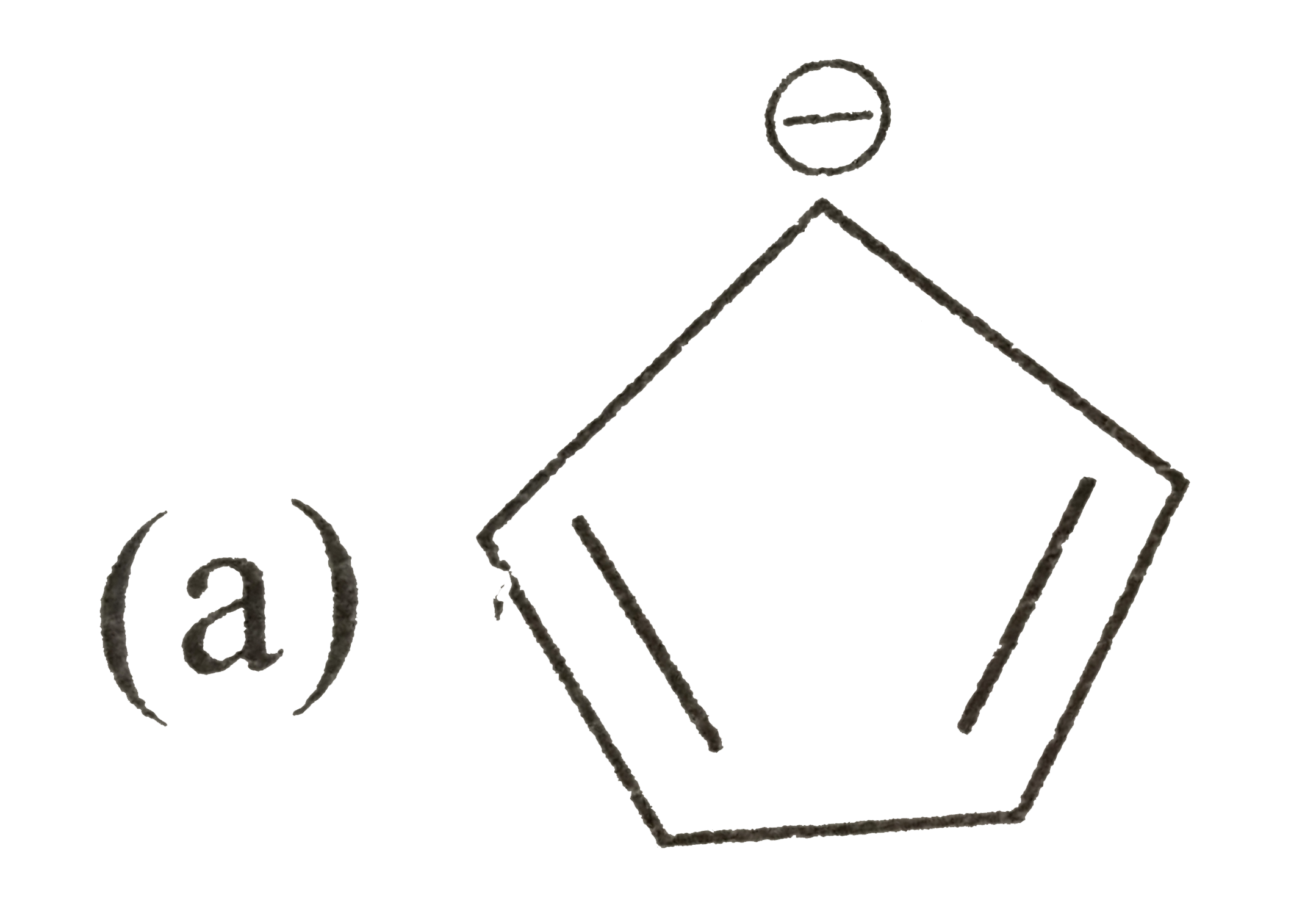A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 2 (Q.26 To Q.50)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 3 (Q.1 To Q.25)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 1 (Q.26 To Q.50)|25 VideosAMINES
HIMANSHU PANDEY|Exercise Subjective Type Problems|5 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Match The Column|6 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AROMATIC HYDROCARBONS-Level 2 (Q.1 To Q.25)
- Which of the following copmpounds will show aromatic character ?
Text Solution
|
- A molecular of benzene has :
Text Solution
|
- Which of the following species is not aromatic ?
Text Solution
|
- Which one of the following compounds is not aromatic ?
Text Solution
|
- The order of stability of compounds X, Y and Z :
Text Solution
|
- Which of the following species are antiaromatic ?
Text Solution
|
- When sodium benzoate is heated with soda lime, the product formed is...
Text Solution
|
- Among the following compounds that can be most readily nitrated is :
Text Solution
|
- Which of the following compounds that can be most readily nitrated ?
Text Solution
|
- Among the compounds the order of decreasing reactivity towards elec...
Text Solution
|
- The following reaction is an example of :
Text Solution
|
- The function of anhydrous AlCl(3) in Friedel- Carfts reaction is to :
Text Solution
|
- The presence of which one of the following group on benzene nucleus a...
Text Solution
|
- Which one of the following compounds give only one isomer upon nitrat...
Text Solution
|
- In the given reaction
Text Solution
|
- Which one of the following aromatic compojunds fails to undergo Fried...
Text Solution
|
- The reaction least likely to occur is :
Text Solution
|
- Consider the following compounds The relative reactivity towa...
Text Solution
|
- In the gives reaction, C(6)H(5)- NH(2) underset("Pyridine")overset(Ac(...
Text Solution
|
- gives :
Text Solution
|