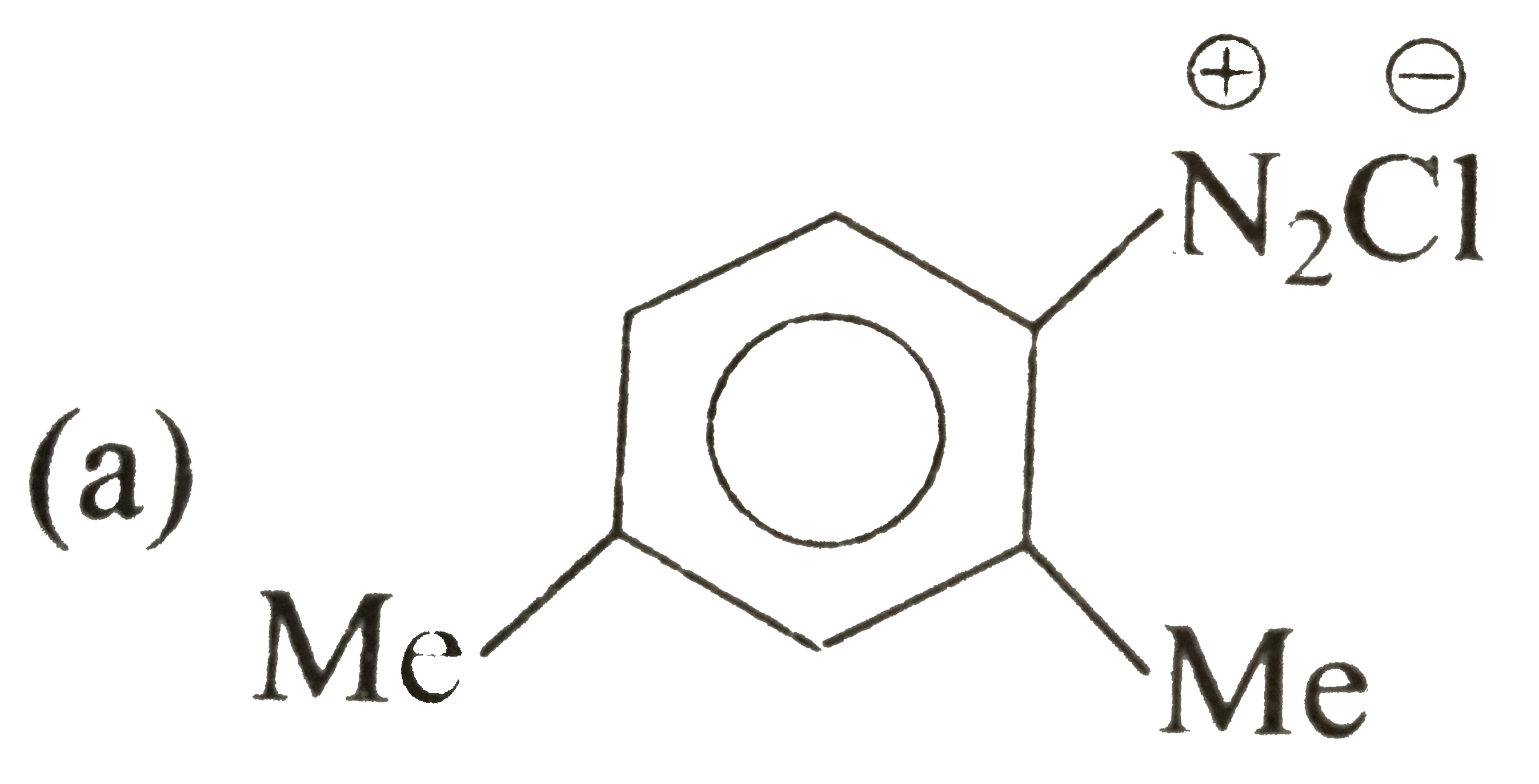
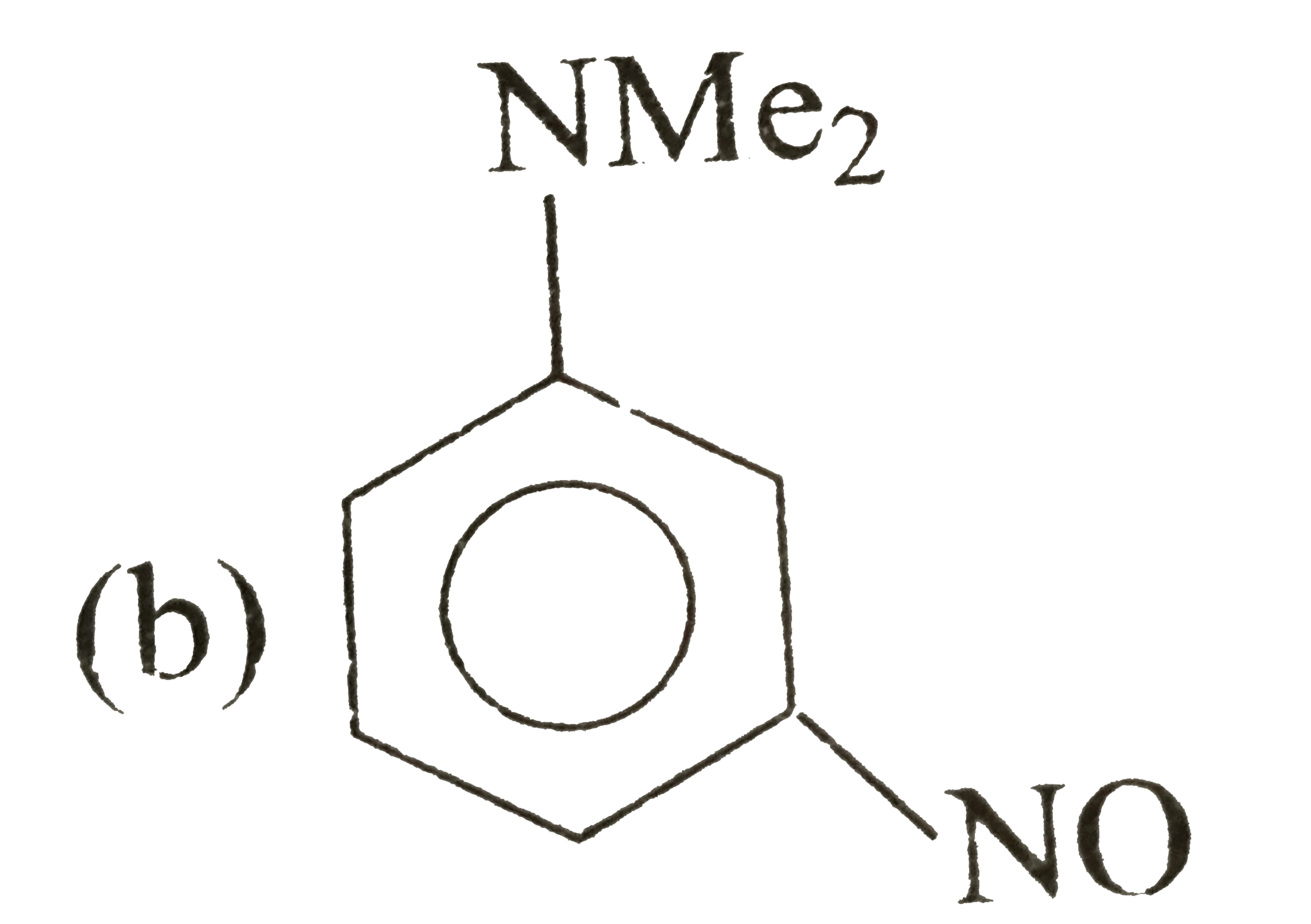
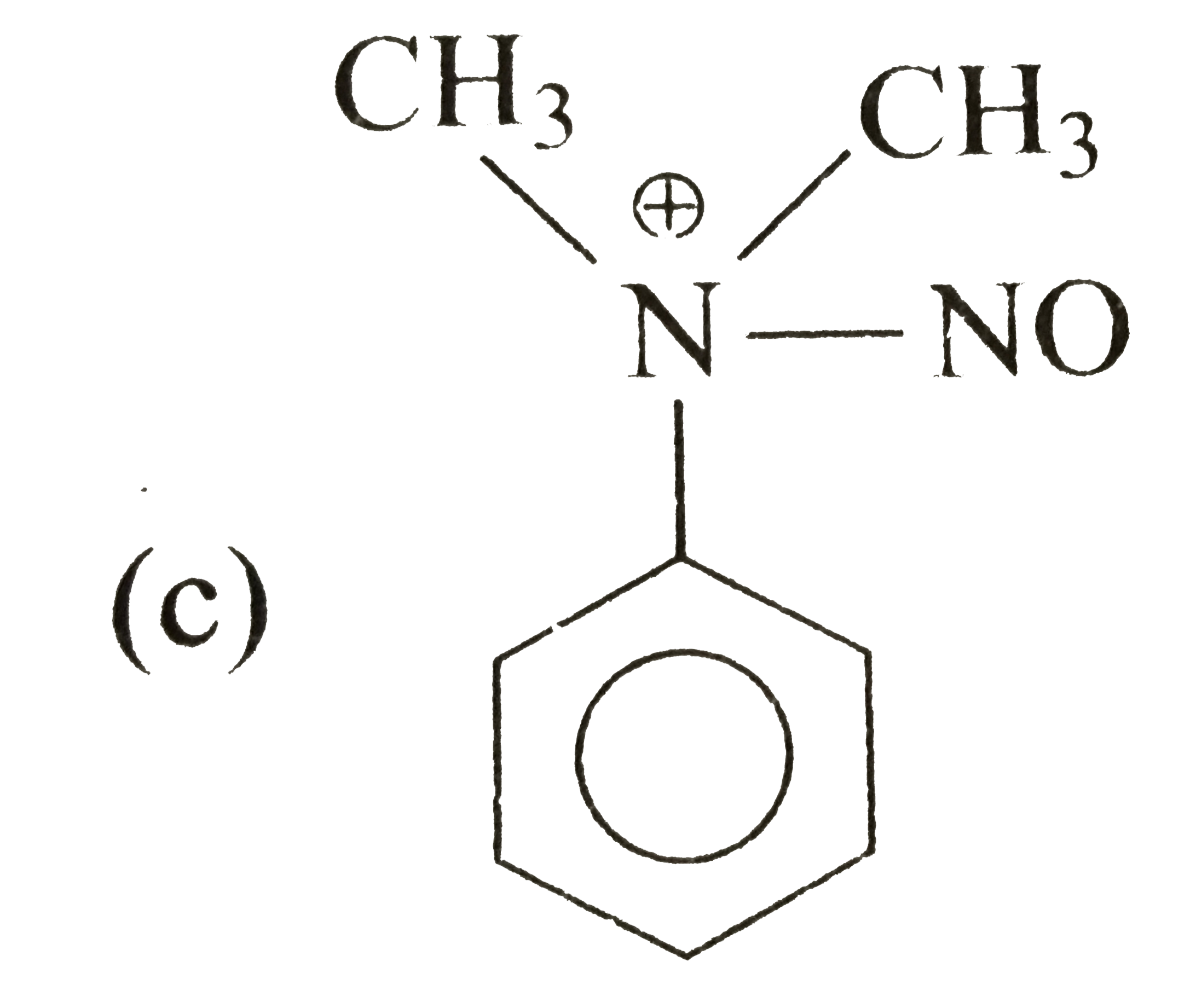
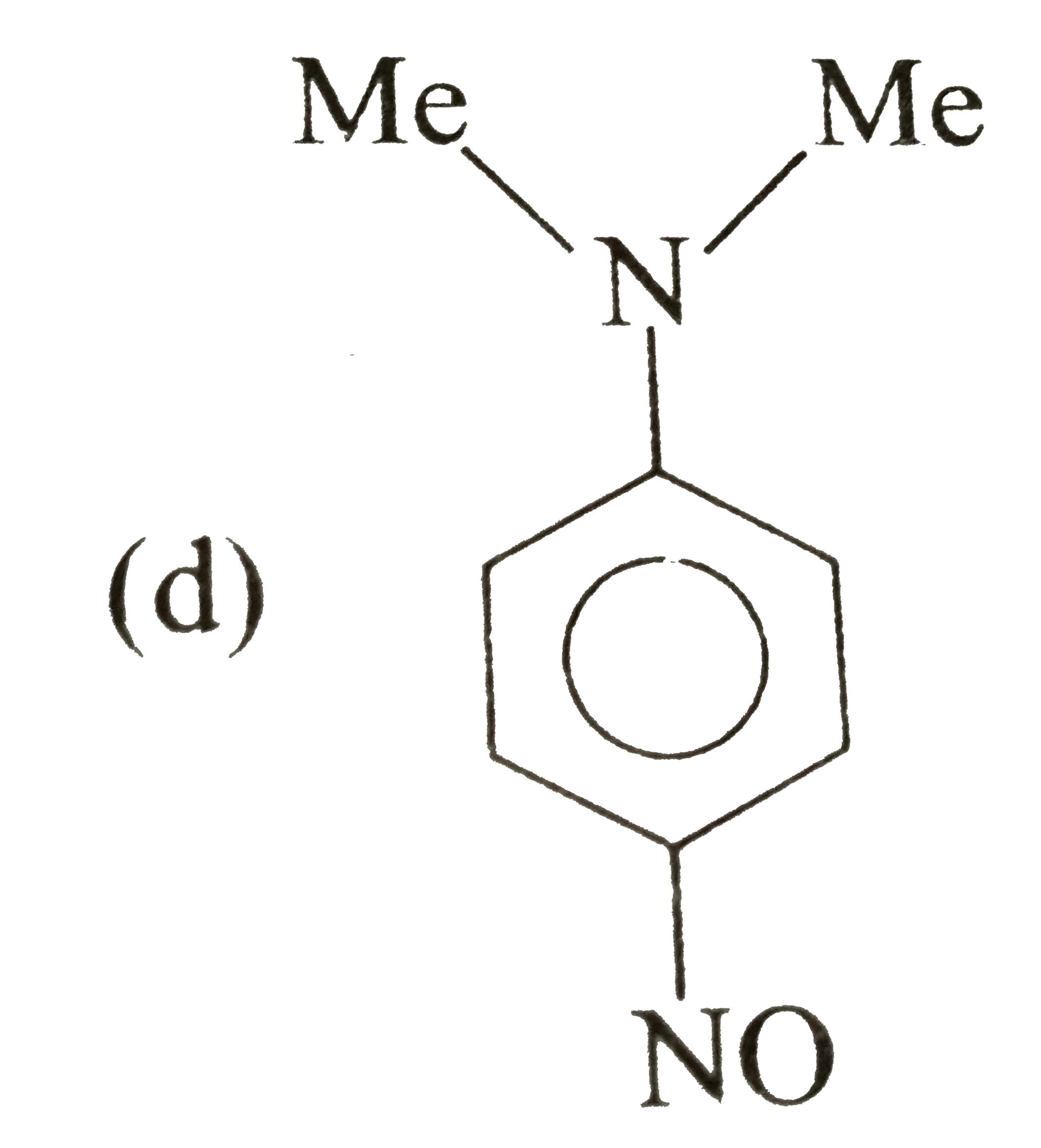
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 3 (Q.26 To Q.50)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 3 (Q.51 To Q.75)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 2 (Q.26 To Q.50)|25 VideosAMINES
HIMANSHU PANDEY|Exercise Subjective Type Problems|5 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Match The Column|6 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AROMATIC HYDROCARBONS-Level 3 (Q.1 To Q.25)
- The major product in the reaction is :
Text Solution
|
- The major product fromed in the reaction :
Text Solution
|
- underset (Conc. H(2)SO(4))overset(Conc. HNO(3))toP underset(Delta)ove...
Text Solution
|
- Which of the following is foomed as a product when m-dinitrobenzene...
Text Solution
|
- Consider the following reactions, overset(Br(2)//FeBr^(3))toPoverse...
Text Solution
|
- Which of the following compounds will form a yellow-coloured oily co...
Text Solution
|
- In Friedel-Carfts acylation reaction the electrophile is :
Text Solution
|
- N,N- Dimethyl aniline react with NaNO(2) and dilute HCl at 0-5^(@...
Text Solution
|
- underset (Hr(2))overset(Br(2))to Xunderset(H(3)PO(2))overset(NaNO(2)//...
Text Solution
|
- Identify the end product (Y) of the following sequence of reaction :
Text Solution
|
- The action of Br(2)//H(2)O on salicylic acid results in the formation...
Text Solution
|
- In the above sequence of reactions which one is not correct ?
Text Solution
|
- The final product of the given reaction sequence :
Text Solution
|
- Which one of the following aryl amine undergoes diazotisation most re...
Text Solution
|
- In the given reaction sequence C(6)H(5)NO(2)overset(Sn//HCl)to A ove...
Text Solution
|
- Which one of the following substrate will not form benzyne when trea...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which one of the following is most stable carbocation ?
Text Solution
|
- Which one of the following is most stable carbanion ?
Text Solution
|
- Nitrantion of the following compound will occur at which position...
Text Solution
|