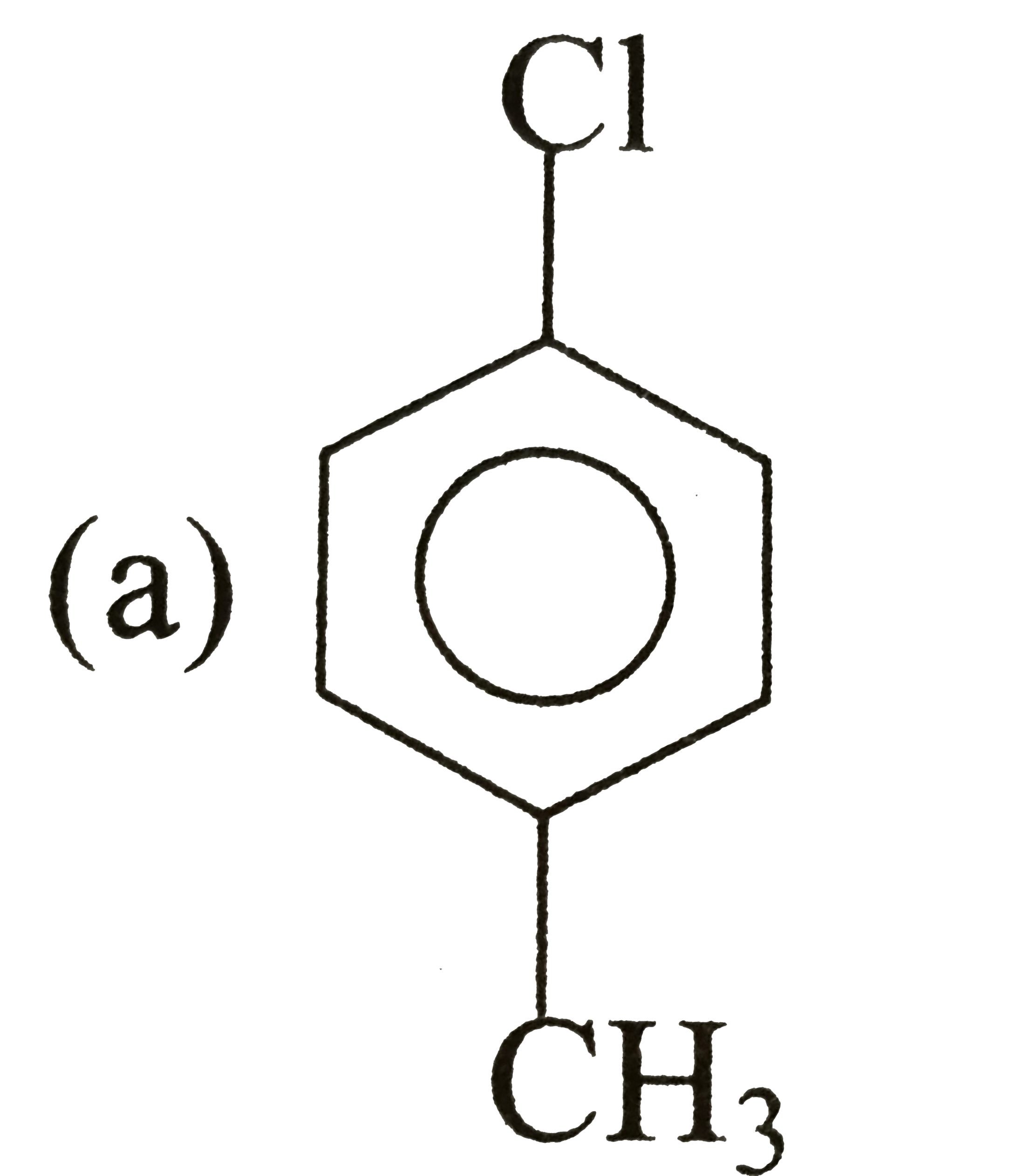
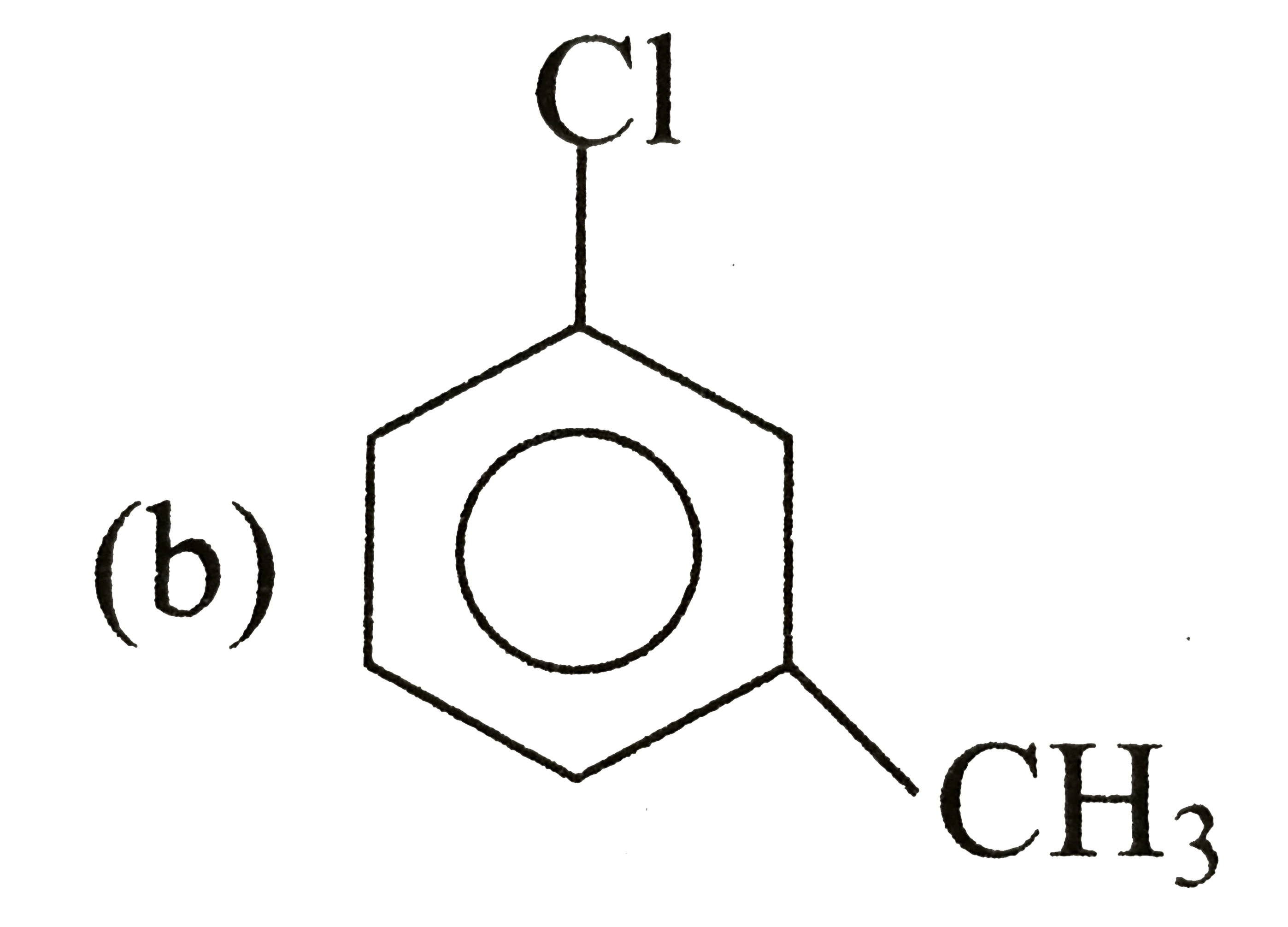
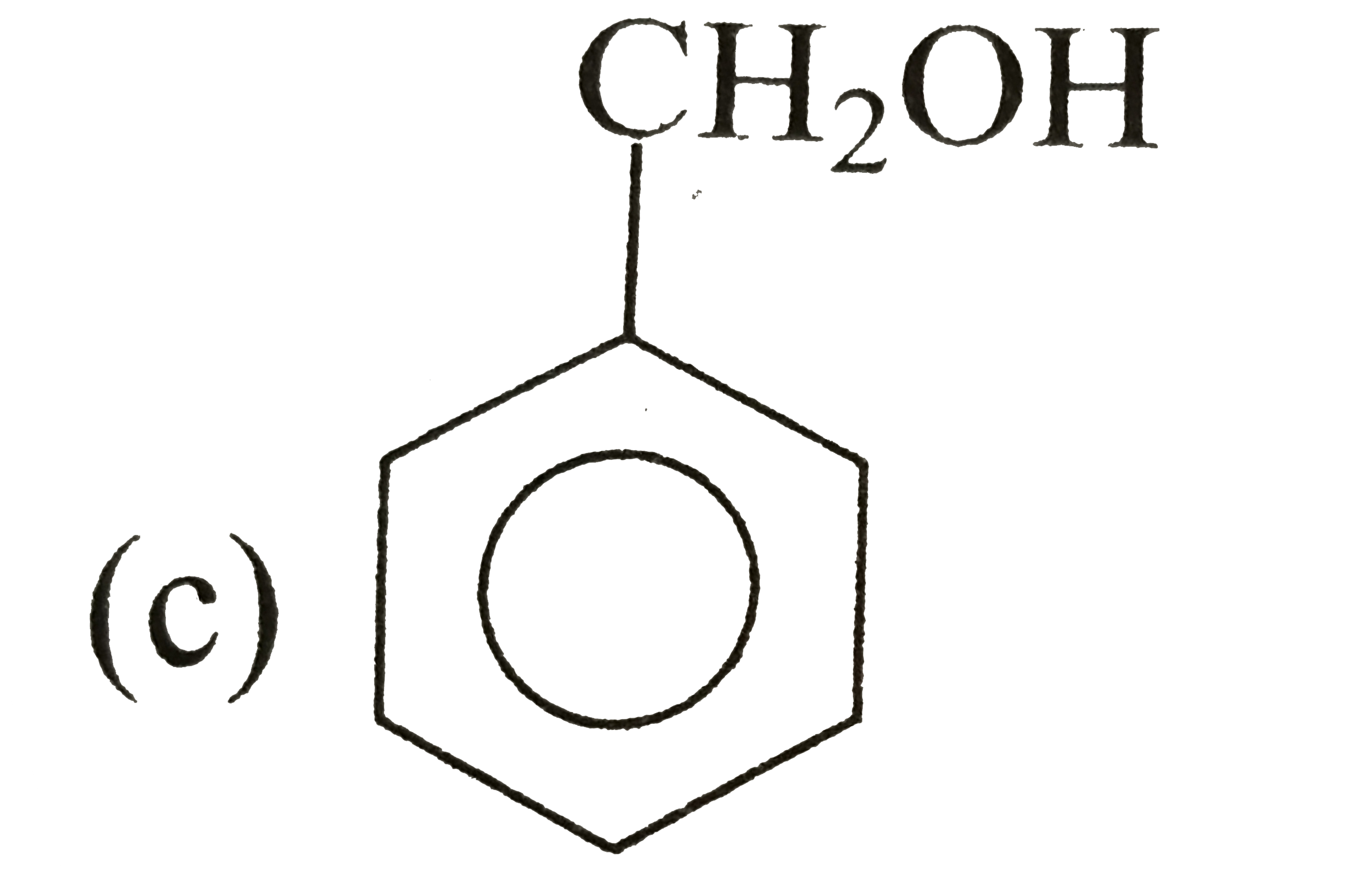
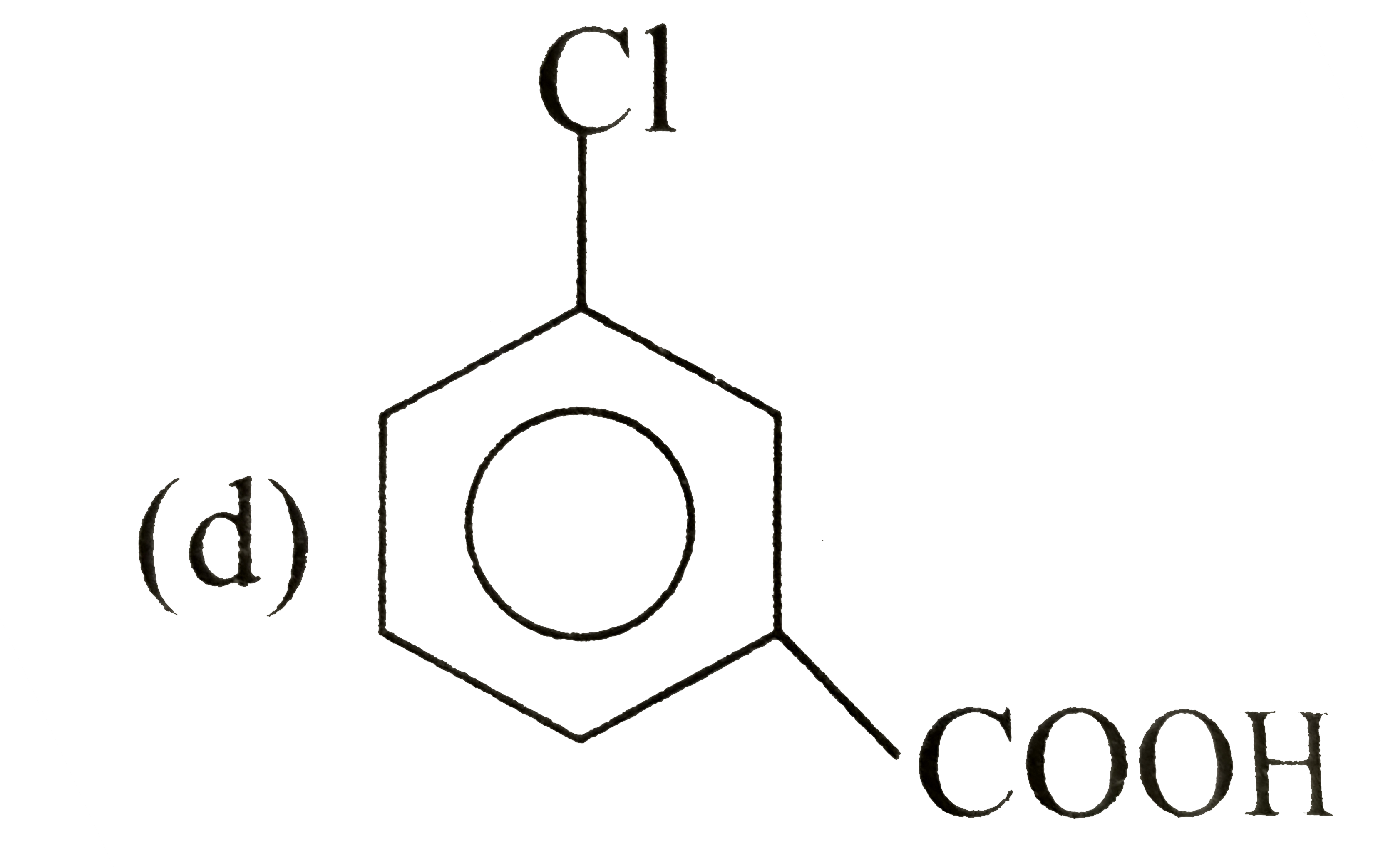
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 3 (Q.151 To Q.160)|10 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise More Than One Correct (Q.1 To Q.25)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 3 (Q.101 To Q.125)|25 VideosAMINES
HIMANSHU PANDEY|Exercise Subjective Type Problems|5 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Match The Column|6 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AROMATIC HYDROCARBONS-Level 3 (Q.126 To Q.150)
- Which product is formed at the end of following reaction ?
Text Solution
|
- The product (Y) of the following sequence of reaction would is :
Text Solution
|
- Consider the following reaction and select correct statement :
Text Solution
|
- Complete the following reaction
Text Solution
|
- . Find out correct product 'Y'.
Text Solution
|
- . Find out correct product 'Z'.
Text Solution
|
- Identify B:
Text Solution
|
- The product B is :
Text Solution
|
- An organic compound A on treatment with CHCl(3) and KOH gives B an...
Text Solution
|
- Compound A (C(7) H(7)Cl) react with aq. KOH at room temperature an...
Text Solution
|
- Arrage the following in decreasing order of reaction with Cl(2)//AlC...
Text Solution
|
- Identify the positoin where, EAS reaction can take place :
Text Solution
|
- The major product of the reaction is :
Text Solution
|
- Product :
Text Solution
|
- Major product of the reaction is :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- The product of the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|