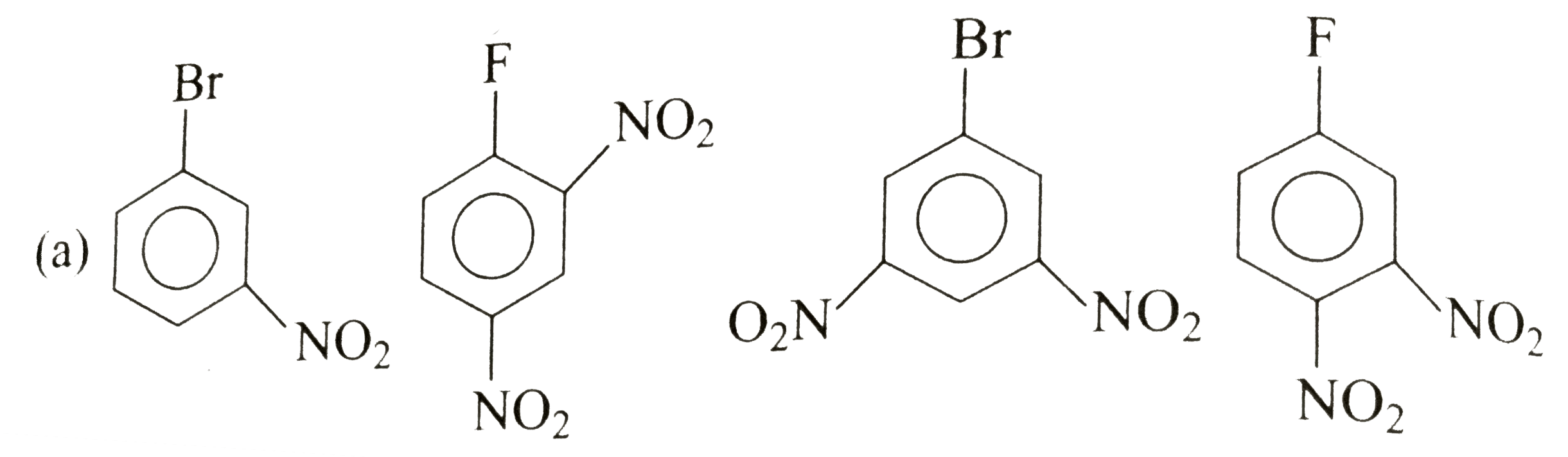
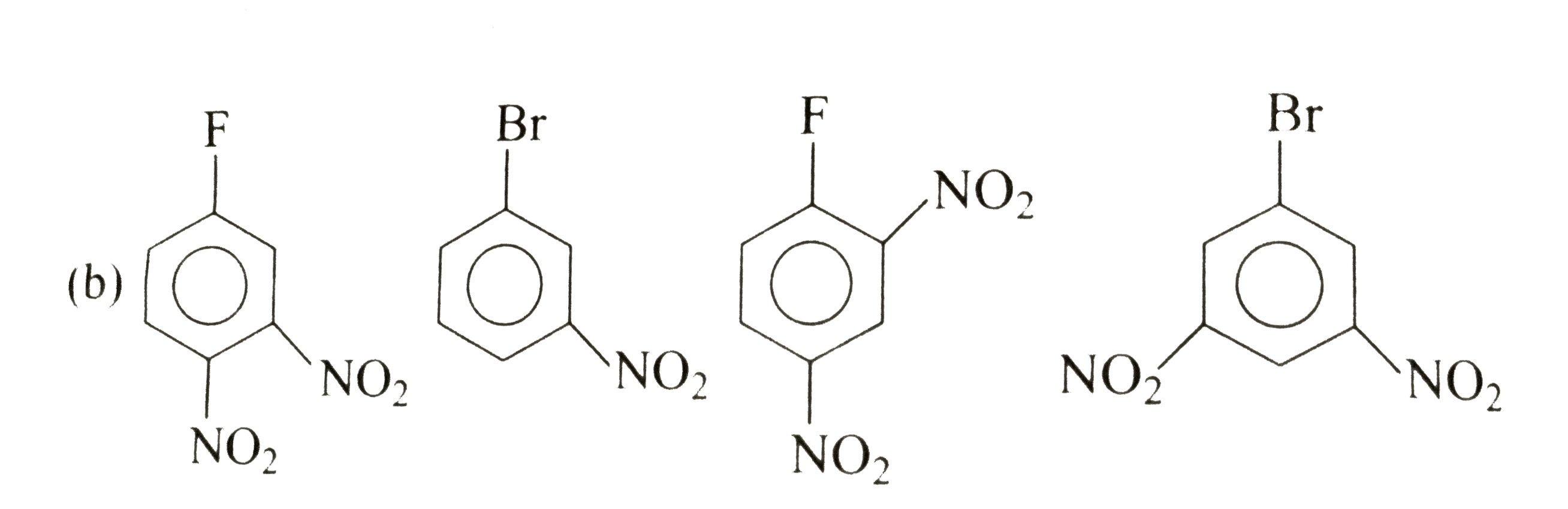
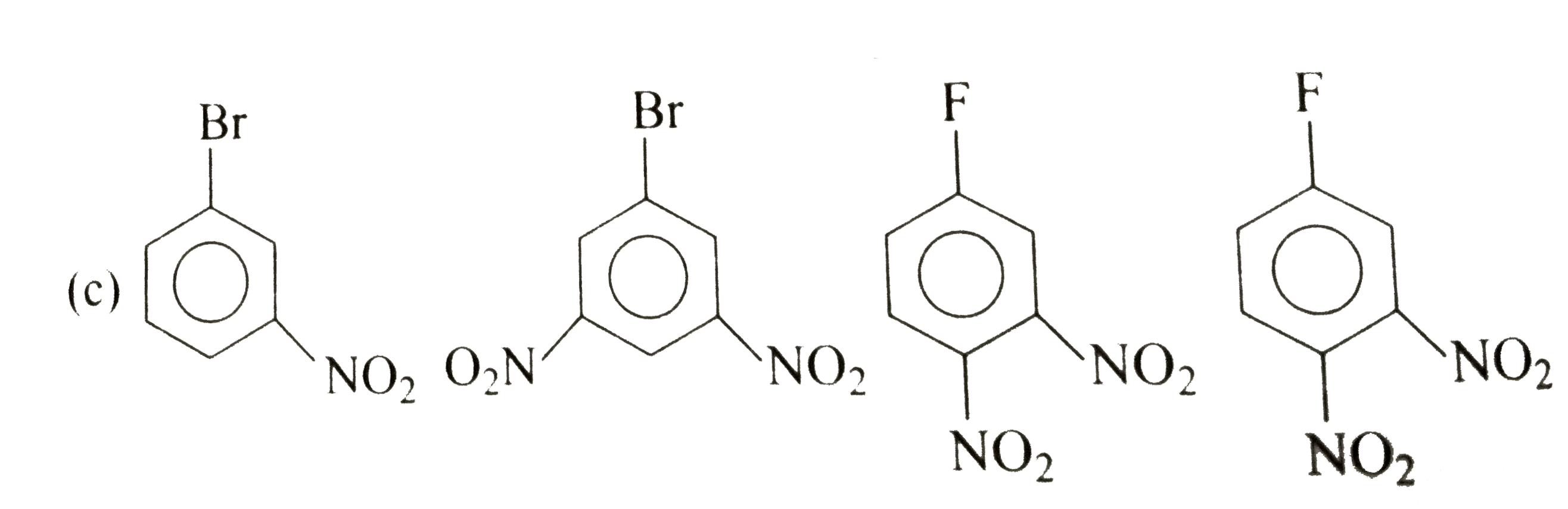
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise More Than One Correct (Q.1 To Q.25)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise More Than One Correct (Q.26 To Q.35)|10 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 3 (Q.126 To Q.150)|25 VideosAMINES
HIMANSHU PANDEY|Exercise Subjective Type Problems|5 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Match The Column|6 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AROMATIC HYDROCARBONS-Level 3 (Q.151 To Q.160)
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which is the best synthesis of
Text Solution
|
- Which is best synthesis of
Text Solution
|
- Which of the following correctly ranks the halides in increasing ...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Find final product of the following reaction :
Text Solution
|
- Find the final product of following sequence of reactions :
Text Solution
|
- Find the final product of following sequence of reactions :
Text Solution
|