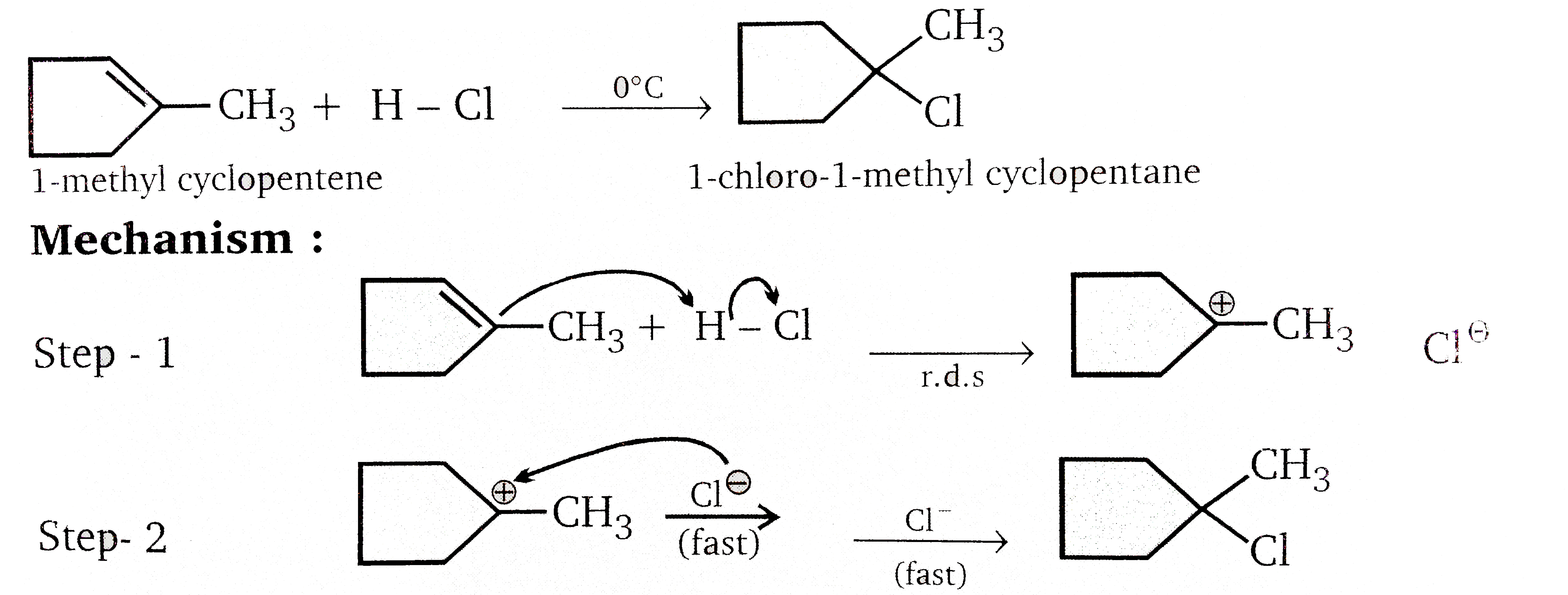
Vladimir Markovnikov rule:
Alkenes undergo electrophilic addition reactions. It is triggered by the acid acting as a electrophile toward `pi`-electrons of the double bond.
Markovnikov's rule states that when an unsymmetrically substituted alkene reacts with a hydrogen halide, the hydrogen atom adds to the carbon that has the greater number of hydrogen, e.g.,
In which of following reactions, carbocation rearrangement is possible?