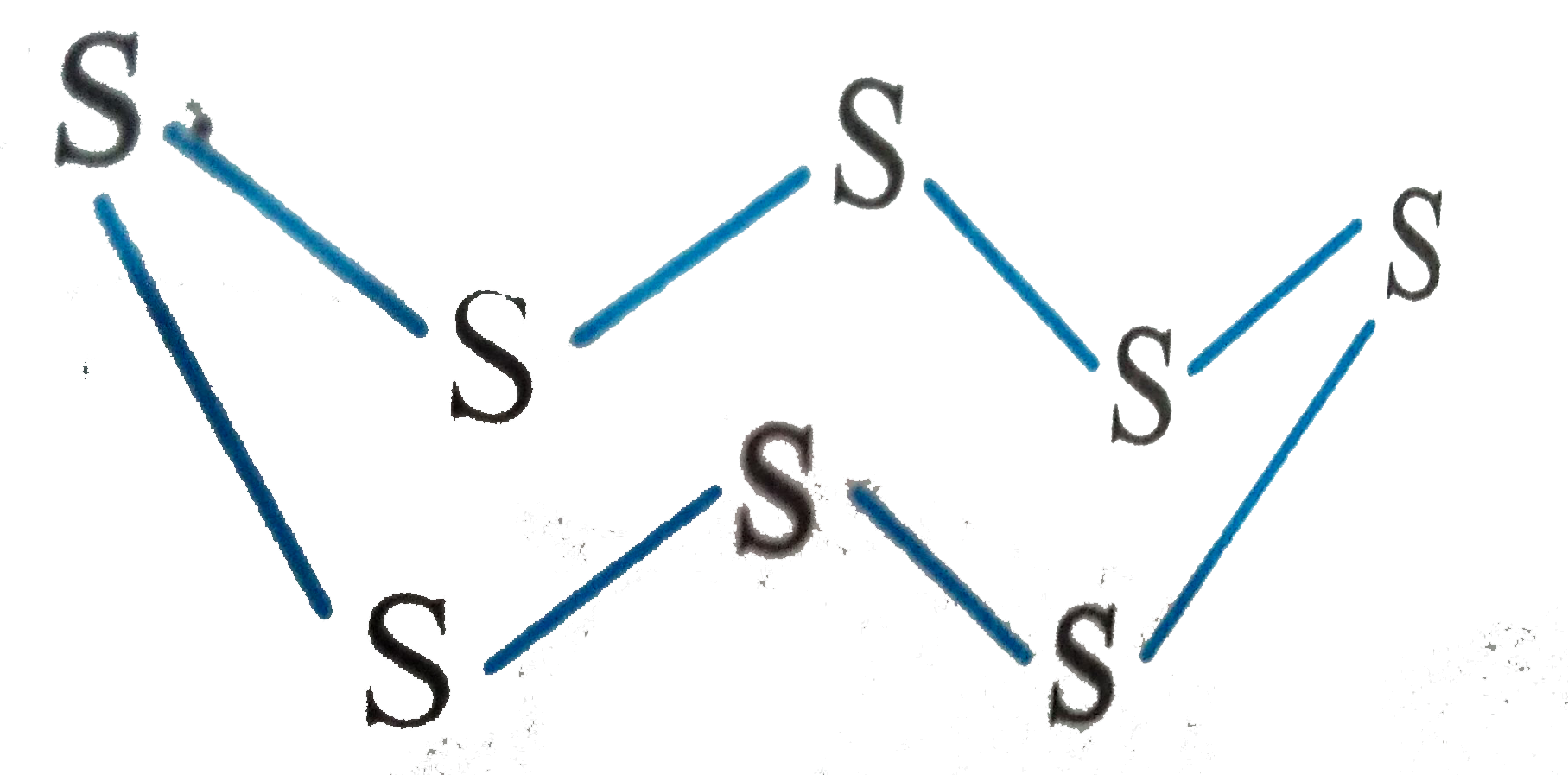
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-SOME BASIC CONCEPTS IN CHEMISTRY STOICHIOMETRY (PART-I)-All Questions
- Which does not undergo comproportionation reaction?
Text Solution
|
- The oxidation number of Oxygen in KO(2) is
Text Solution
|
- The oxidation number and covalency of sulphur in the sulphur molecule ...
Text Solution
|
- Na(2)SO(3)+H(2)O(2) rarr Na(2)SO(4) +H(2)O, in reaction
Text Solution
|
- In which of the following compounds, sulphur atom has different oxida...
Text Solution
|
- Change in oxidation number is maximum in the following reaction
Text Solution
|
- Which of the following are neutral oxide?
Text Solution
|
- The oxidation state of N in HNO(4) is
Text Solution
|
- Which of the following can acts as a reducing agent ?
Text Solution
|
- Which of the following is displacement reaction :
Text Solution
|
- N kg^(-1) is the unit of :
Text Solution
|
- Prefix zepto and femto stands for which multiples ?
Text Solution
|
- The number of significant figures in N(0) = 6.022 xx 10^(23) (Avogadro...
Text Solution
|
- The correctly reported answer of the addition of 4.523, 2.3 and 6.24 w...
Text Solution
|
- The answer of the calculation (2.568 xx 5.8)/(4.168) in significant fi...
Text Solution
|
- How many significant figures are present in the following ? (i) 0.00...
Text Solution
|
- 314.000 has how many significant figures.
Text Solution
|
- The correctly reported difference of 16.4215 and 6.01 will have signif...
Text Solution
|
- After rounding off 1.235 and 1.225, we will have their answer respecti...
Text Solution
|
- In the formation of SO(2) and SO(3) the ratio of the weights of oxygen...
Text Solution
|