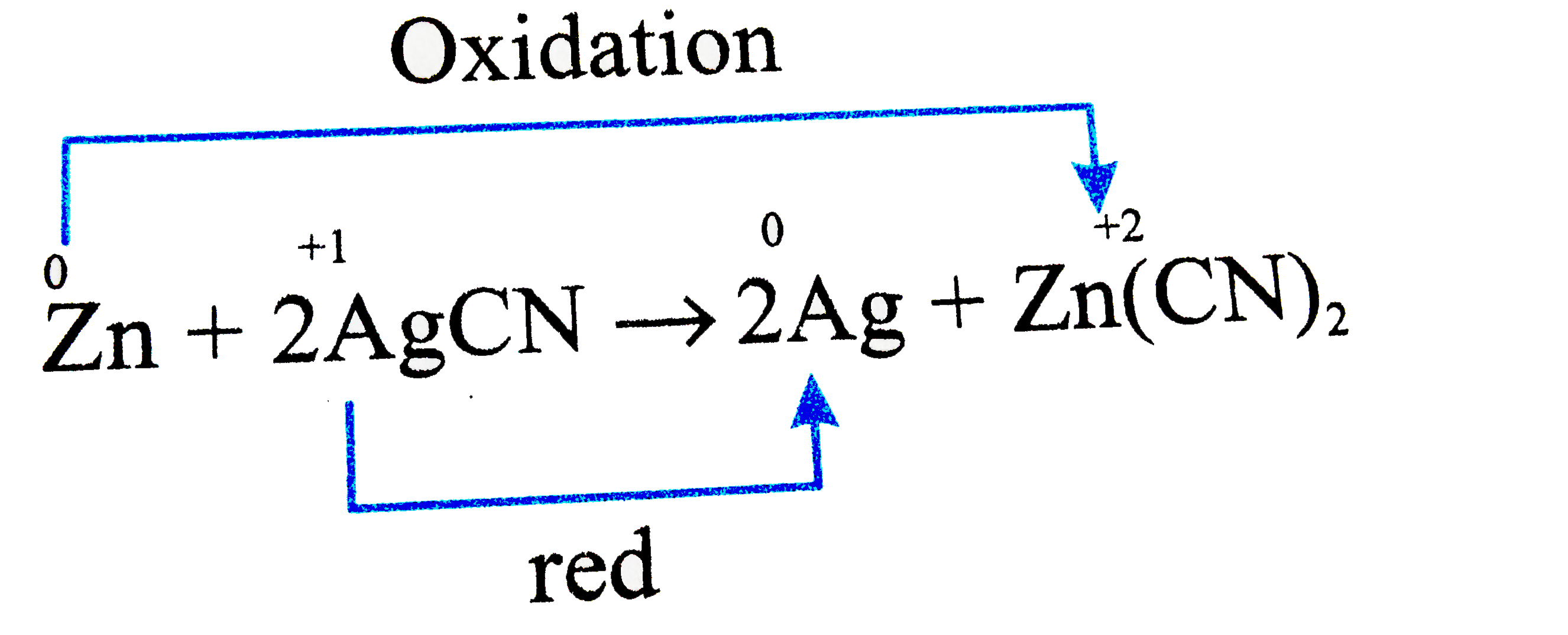
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-SOME BASIC CONCEPTS IN CHEMISTRY STOICHIOMETRY (PART-I)-All Questions
- Oxidation number of 'Co' in Hg[Co(SCN)(4)]
Text Solution
|
- Oxygen can show positive oxidation state in its compounds with
Text Solution
|
- which of the following is a redox reaction ?
Text Solution
|
- The number of significant figures in pi are
Text Solution
|
- Given the number: 161 cm, 0.161 cm, 0.0161 cm. The number of significa...
Text Solution
|
- If water sample are taken from sea, rivers or lake, they will be found...
Text Solution
|
- The percentage of hydrogen in water and hydrogen peroxide is 11 .1 and...
Text Solution
|
- The density of water is 1gm//ml. The volume of water drop is 1.8 ml. T...
Text Solution
|
- Increasing order of number of moles of the species (i) 3grams of No (i...
Text Solution
|
- 0.44 g of colourless oxide of nitrogen occupies 224 ml at STP. The mol...
Text Solution
|
- How many moles of magnesium phosphate, Mg(3)(PO(4)(2) will contain 0.2...
Text Solution
|
- Volume of a gas at STP is 1.12xx10^(-7) c c. Calculate the number of m...
Text Solution
|
- Number of electrons in 1.8 grams of H(2)O are
Text Solution
|
- From 320 mg. of O(2), 6.023 xx 10^(20) molecules are removed, the no. ...
Text Solution
|
- An oxide of nitrogen has a molecular weight 92. Find the total number ...
Text Solution
|
- Number of moles of KI required to prepare 0.4 moles of K(2)Hgl(4), whe...
Text Solution
|
- How many water molecules are there in one drop pf water (volume 0.0018...
Text Solution
|
- 1.5g CdCl(2) was formed to contain 0.9g Cd. Calculate the atmic weight...
Text Solution
|
- The vapour density of a tribasic acid is x. the equivalent mass of tha...
Text Solution
|
- The equivalent weight of divalent metal is 31.82. The weight of a sing...
Text Solution
|