A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-SOME BASIC CONCEPTS IN CHEMISTRY STOICHIOMETRY (PART-I)-All Questions
- Versene, a chelating agent having chemical formula C(2)H(4)N(2)(C(2)H...
Text Solution
|
- One mole of a mixture of CO and CO(2) requires exactly 20g of NaOH in ...
Text Solution
|
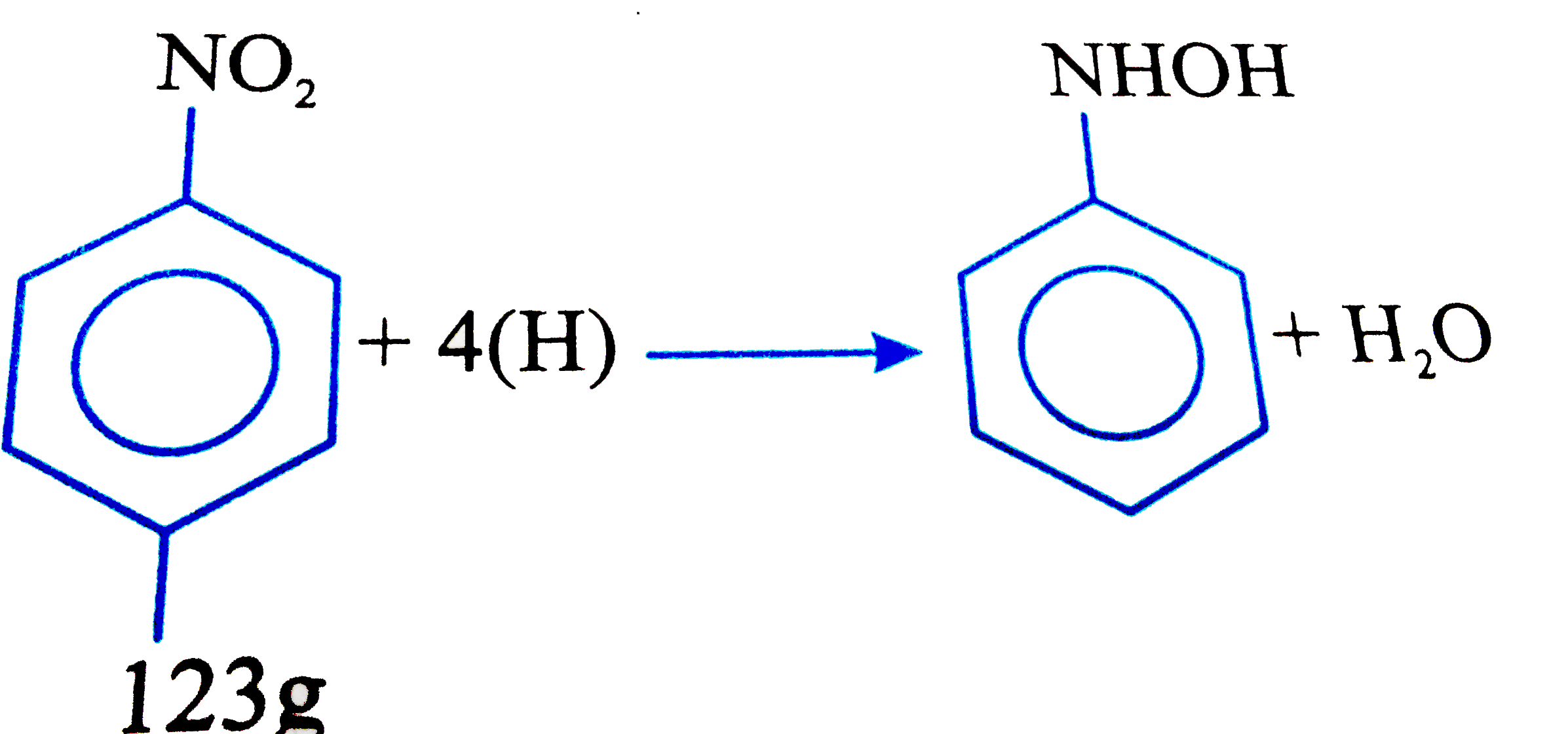
- 1.23 g of Nitro Benzene on reduction with Zn + NH(4)Cl then its gives ...
Text Solution
|
- A mixture of HCOOH and H(2)C(2)O(4) is heated with conc. H(2)SO(4). Th...
Text Solution
|
- When burnt in air, a 12.0 g mixture of carbon and sulphur yields a mix...
Text Solution
|
- The oxide of a metal contains 40% of oxygen. The valency of metal is 2...
Text Solution
|
- Cuprous sulphide and silver sulphide are isomorphous. The atomic mass ...
Text Solution
|
- A sample of 1.0g of solid Fe(2)O(3) of 80% purity is dissolved in a mo...
Text Solution
|
- A gaseous hydrocarbon gives upon combustion 0.72g of water and 3.08g o...
Text Solution
|
- 10 " mL of " a gaseous organic compound containing C, H and O only was...
Text Solution
|
- A hydrocarbon contains 10.5 g of carbon per gram of hydrogen. 1 L of v...
Text Solution
|
- When K(2)Cr(2)O(7) is heated with conc. H(2)SO(4) and soluble chloride...
Text Solution
|
- A 20 mL mixture of CO, CH4, and Helium (He) gases is exploded by an el...
Text Solution
|
- 7.36g of a mixture of KCI and KI was dissolved in H(2)O to prepare 1 l...
Text Solution
|
- Equal weights of Zn metal and iodine are mixed together and I(1) is co...
Text Solution
|
- A hydrocarbon 'X' have 81% of carbon. Volume of CO(2) liberated at 298...
Text Solution
|
- Gastric juice contains 3g HCl per liter. If a person produces 2.5 L of...
Text Solution
|
- 50.0 kg of N(2)(g) and 10.0 kg of H(2)(g) are mixed to produce NH(3)(g...
Text Solution
|
- Calcium carbonate reacts with aqueous HCl to give CaCl(2) and CO(2) ac...
Text Solution
|
- 3.92g of ferrous ammonium sulphate (FAS) react completely with 50 mlN/...
Text Solution
|