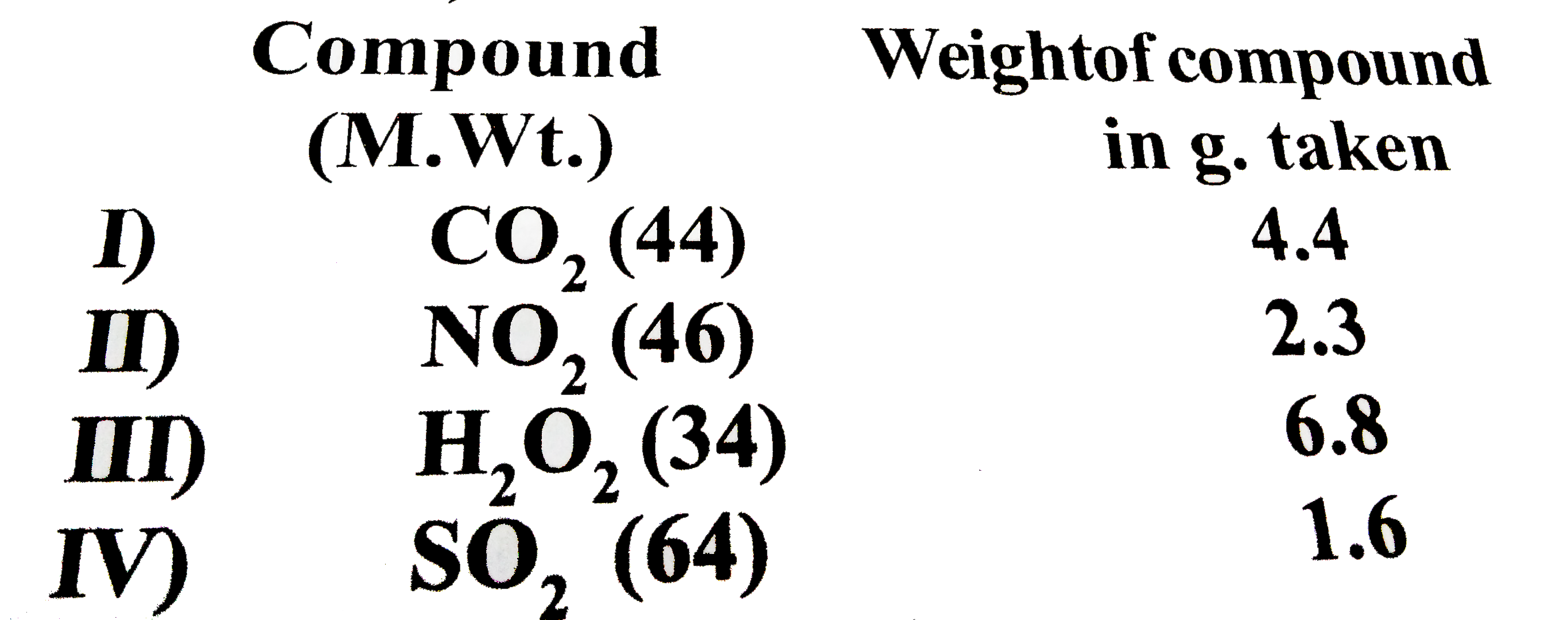A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-SOME BASIC CONCEPTS IN CHEMISTRY STOICHIOMETRY (PART-I)-All Questions
- Irrespective of the source, pure sample of water always yields 88.89% ...
Text Solution
|
- The ratio of the mass of C-12 atom to that of an atom of element X (wh...
Text Solution
|
- Study the following table: Which two compounds have least weight of ox...
Text Solution
|
- Which of the following contain 9 xx 10^(23) oxygen atoms?
Text Solution
|
- Number of gram atoms of oxygen present in 0.3 mole of (COOH)(2).2H(2)O...
Text Solution
|
- No. of moles of water in 488.6 gms of BaCl(2).2H(2)O are (molecular we...
Text Solution
|
- Equivalent weight of metal 'M' is 12. Equivalent weight of Y in the co...
Text Solution
|
- The oxide of an element possesses the formula MO(2). If the equivalent...
Text Solution
|
- 3 g of an oxide of a metal is converted completely to 5 g chloride. Eq...
Text Solution
|
- A compound contains 28% N and 72% of a metal by weight . Three atoms o...
Text Solution
|
- An organic compound contains 40%C, 6.6%H. The empirical formula of the...
Text Solution
|
- X and Y are two different elements having their atomic masses in 1:2 r...
Text Solution
|
- When 4g of iron is burnt to ferric oxide at constant pressure, 29.28KJ...
Text Solution
|
- X' grams of calcium carbonate was completely burnt in air. The weight ...
Text Solution
|
- When oxalic acid is heated with concentrated H(2) SO(4) it produces
Text Solution
|
- Which of the following solutions has the highest normality ?
Text Solution
|
- 12 ml of 0.25 N H(2)SO(4) is neutralised with 15 ml of sodium hydroxid...
Text Solution
|
- The specific gravity of H(2)SO(4) is 1.8 g// c c and this solution is...
Text Solution
|
- What volume of 75%H(2)SO(4) by mass is required to prepare 1.5 litres ...
Text Solution
|
- 4.9 g of H(2)SO(4) is present in 100 mL of the solution. What is the m...
Text Solution
|