A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-JEE MOCK TEST 20-CHEMISTRY
- The set representing the correct order of ionic radii is
Text Solution
|
- Gem dihalides on treatment with alcoholic KOH give
Text Solution
|
- Which of the following has longest C- O bond length? (Free C - O bond ...
Text Solution
|
- MF + XeF(4) rarr M^(+) A^(-) (M^(+)- alkali metal cation) The state of...
Text Solution
|
- Polystyrene , dacron and orlon are classified respectively as
Text Solution
|
- Which of the acids cannot be prepared by Grignard reagent?
Text Solution
|
- pH of a 100 cc solution is 2. It will not change if
Text Solution
|
- Determine the order of basic stregth of the given molecules
Text Solution
|
- Four successive members of the first row transition elements are liste...
Text Solution
|
- The concentration in g//L of a solution of cane sugar (Molecular weigh...
Text Solution
|
- CsCl crystallises in body centred cubic lattice. If 'a' its edge lengt...
Text Solution
|
- Phenol can be distinguished from ethanol by the following reagents exc...
Text Solution
|
- Which of the following is an intensive property?
Text Solution
|
- For the following three reaction 1, 2 and 3, equilibrium constants are...
Text Solution
|
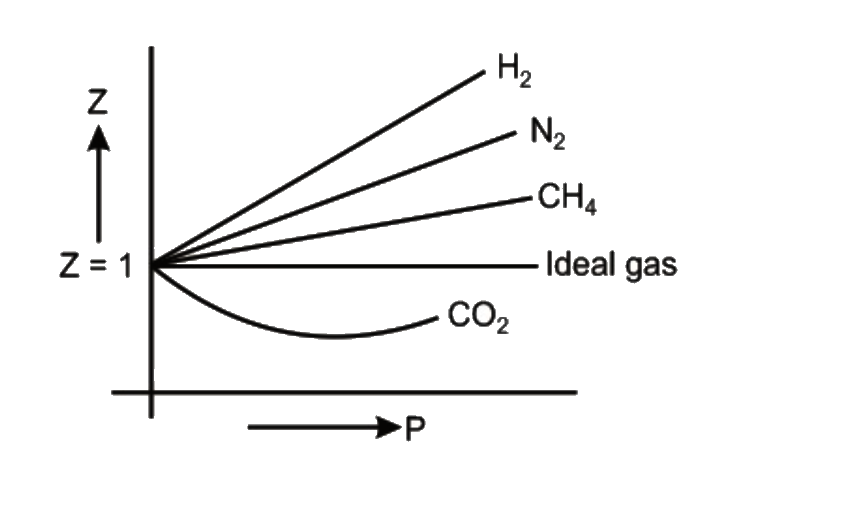
- Consider the graph between compressibility factor Z and pressure P, ...
Text Solution
|
- How many of the following species are related to Hall's process of pur...
Text Solution
|
- The dipole moment of HBr is 1.6 xx 10^(-30) cm and interatomic spaci...
Text Solution
|
- How many of the following acids will show higher reactivity towards es...
Text Solution
|
- Consider an electrochemical cell : A(s)|A^(n+) (aq. 2M)||B^(2n+) (aq...
Text Solution
|
- The electrophile involved in above reaction has lone pair of electrons...
Text Solution
|