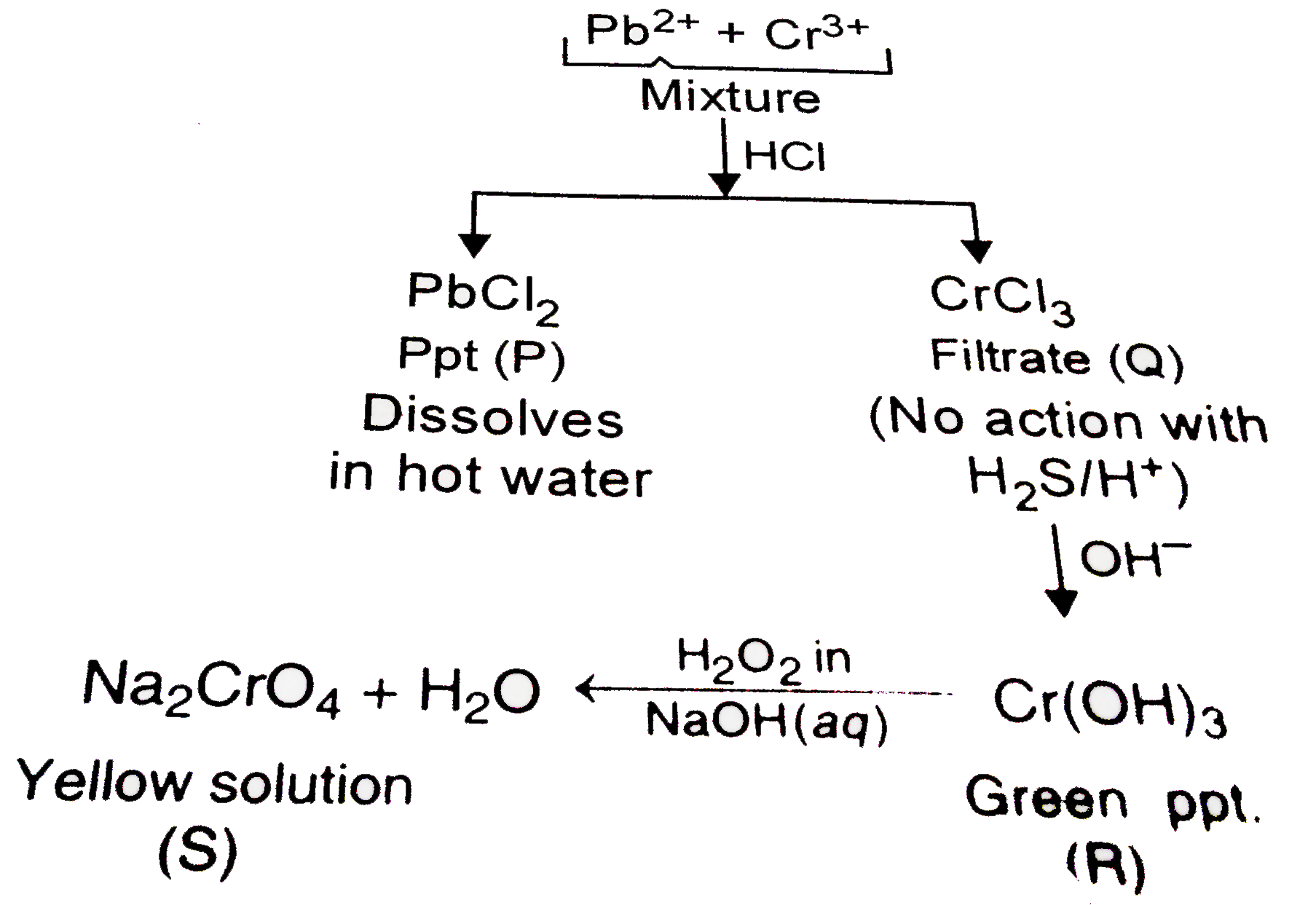A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-APPENDICES-Multiple choice
- CB(2) layer gives a test for
Text Solution
|
- Upon treatment with ammoniacal H(2)S solution, the metal ion precipita...
Text Solution
|
- Match the list I with list II and select the correct answer using the ...
Text Solution
|
- A yellow turbidity, sometimes appears on passing H(2)S gas ever in the...
Text Solution
|
- X' is a colourless salt giving following reactions : 'X' can be
Text Solution
|
- An aqueous solution of a mixture of two inorganic salts when treated w...
Text Solution
|
- An aqueous solution of a mixture of two inorganic salts when treated w...
Text Solution
|
- Among PbS, CuS, HgS, MnS, Ag(2)S, NiS, CoS, Bi(2)S(3) and SnS(2), tota...
Text Solution
|
- The incorrect statement in respect of chromyl chloride test is
Text Solution
|
- When I(2) is passed through KCl, KF and KBr solution
Text Solution
|
- Among the following observation, the correct one that differentiates b...
Text Solution
|
- The pair (s) where both the ions are precipitated upon passing H(2)S g...
Text Solution
|
- In the presence of dilute HCl, H(2)S results in the precipitation of g...
Text Solution
|
- Consider the following salts : NaCl, HgCl(2), CuCl(2), CuCl and AgCl...
Text Solution
|
- The hottest region of Bunsen flame shown in the figure below is :
Text Solution
|
- compound X is tested and the results are shown in the following table....
Text Solution
|
- The reagent (s) that can selectively precipitate S^(2-) from a mixture...
Text Solution
|
- HgCl(2) and I(2) both when dissolved in water containing I^(-)ions, th...
Text Solution
|
- When BaCl(2) is added to an aqueous salt solution, a while precipitate...
Text Solution
|
- The correct options (s) to distinguish nitrate salts of Mn^(2+) and Cu...
Text Solution
|