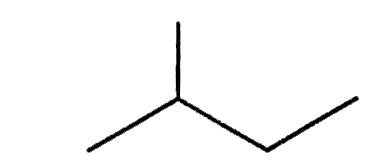
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-NOMENCLATURE OF ORGANIC COMPOUNDS-MCQs
- Write the IUPAC name of the compound (A) in which the molar rations of...
Text Solution
|
- A hydrocarbon of molecular weight 72gmol^(-1) has a 2-methyl group. Wh...
Text Solution
|
- (a) Sketch the carbon skeleton of 3-ethyl-2,5-hexadiene and point out ...
Text Solution
|
- Write the structural formula of a compound of molecular formula C(4)H(...
Text Solution
|
- Write the structural formulae of the following compounds along with th...
Text Solution
|
- The IUPAC name of the given compound will be :
Text Solution
|
- The IUPAC name of the compound
Text Solution
|
- The IUPAC name of the compound is
Text Solution
|
- The IUPAC name of {:(CH(2)-C-CH-CH(2)-C-NH(2)),("| || ...
Text Solution
|
- The IUPAC name of the following compound
Text Solution
|
- The IUPAC name of the compound CH(3)CH(2)underset(underset("SH")(|))...
Text Solution
|
- Number of primary, secondary and tertiary hydrogen in the following ar...
Text Solution
|
- Which among the following in an example of tertiary acid amide ?
Text Solution
|