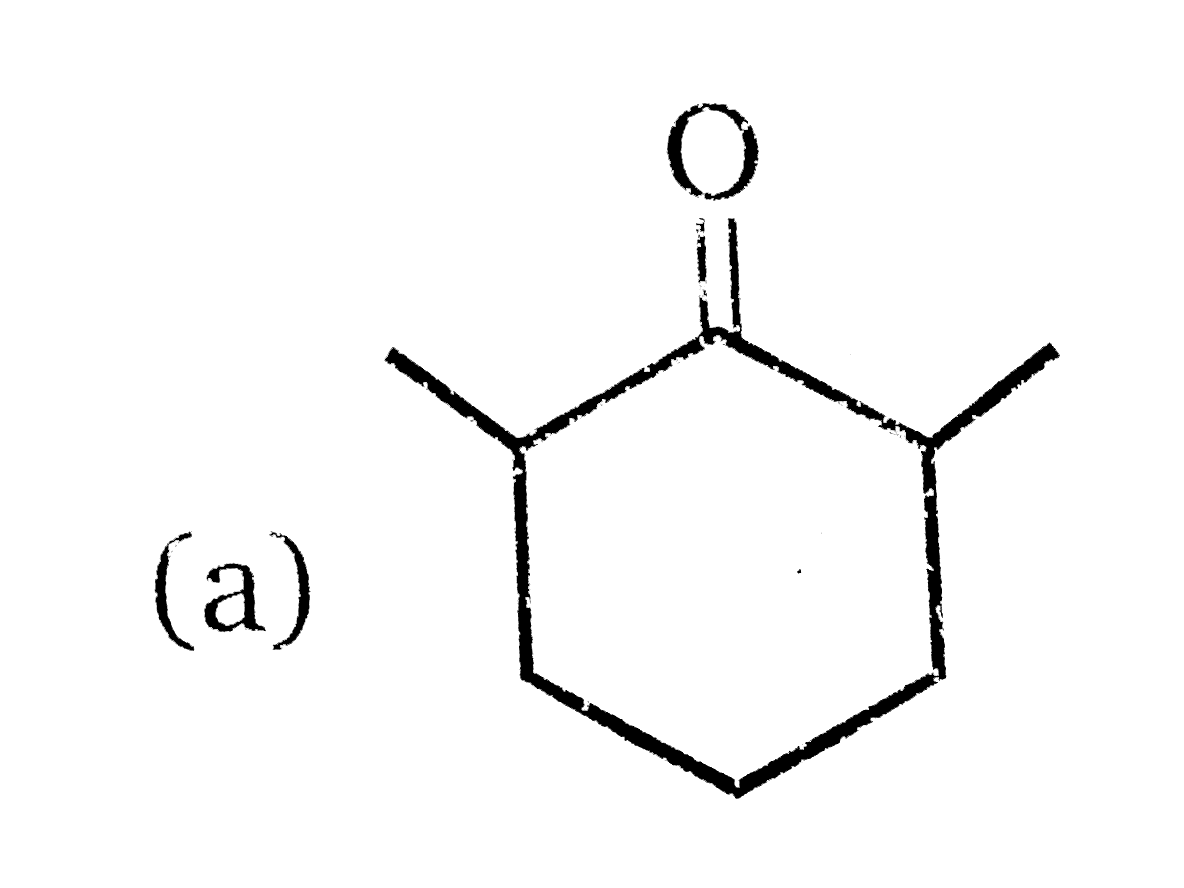
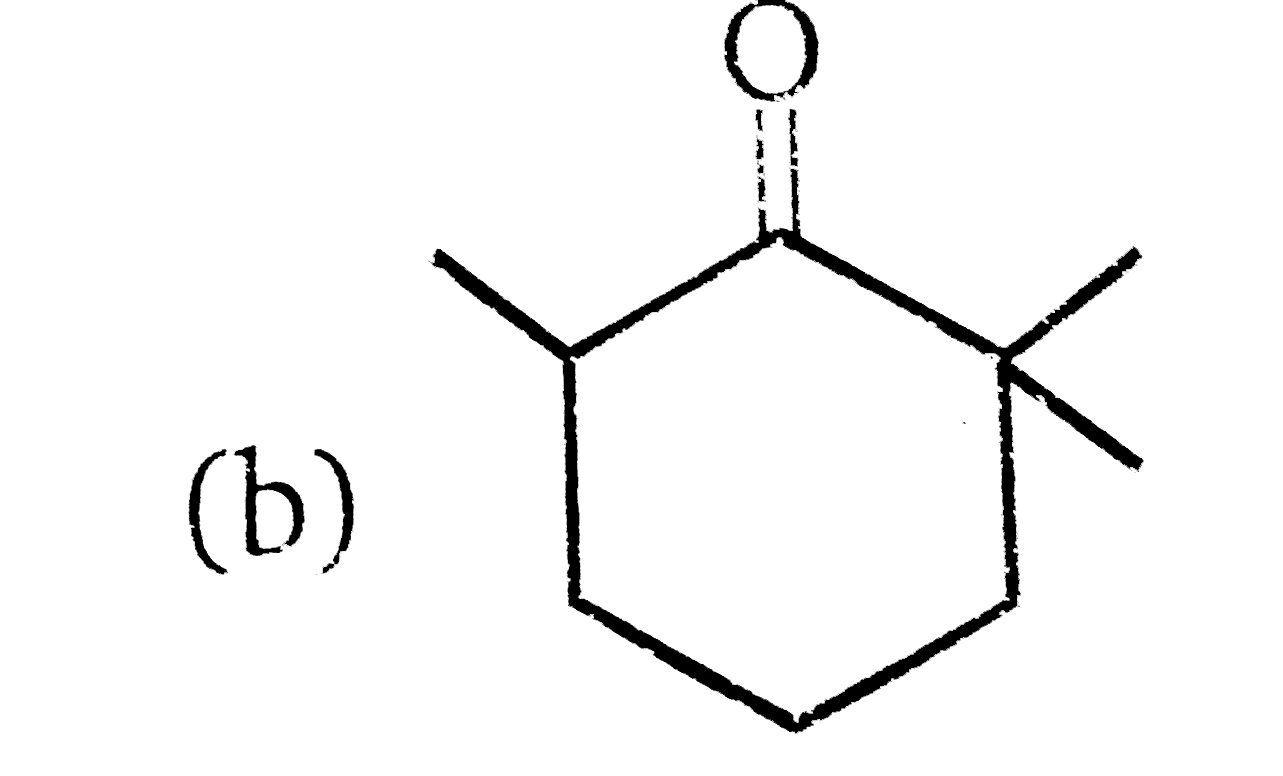
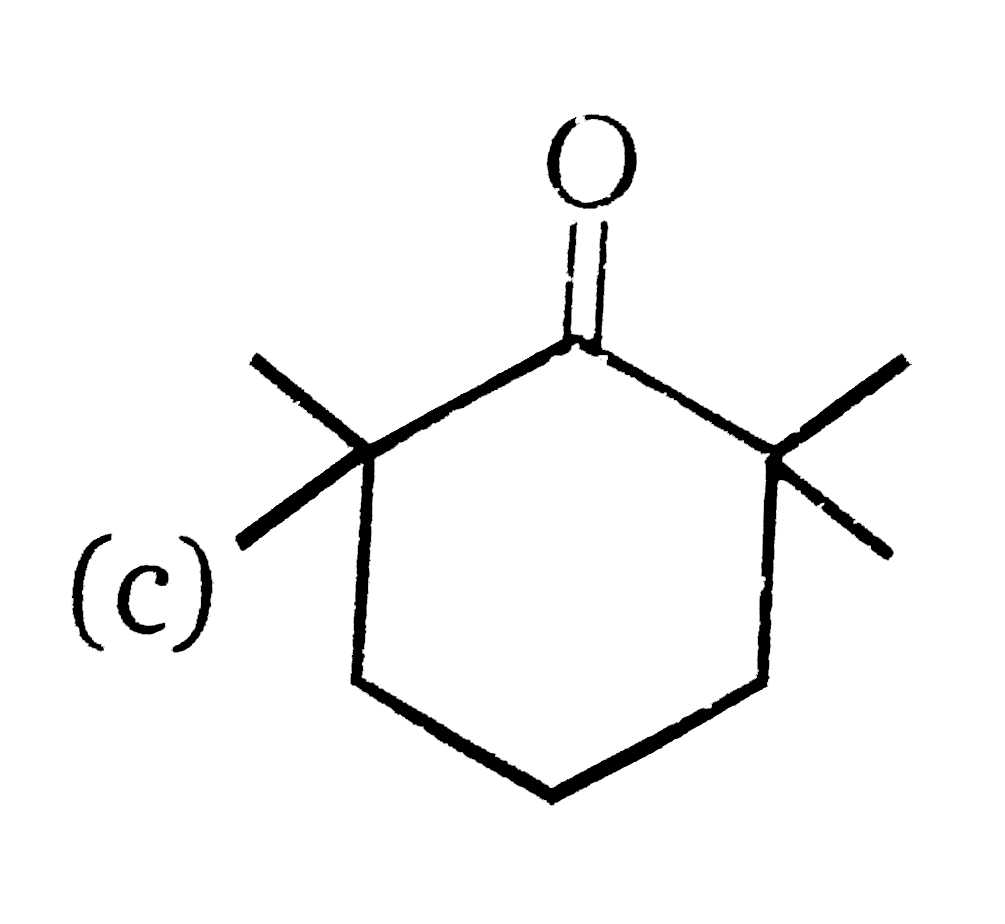
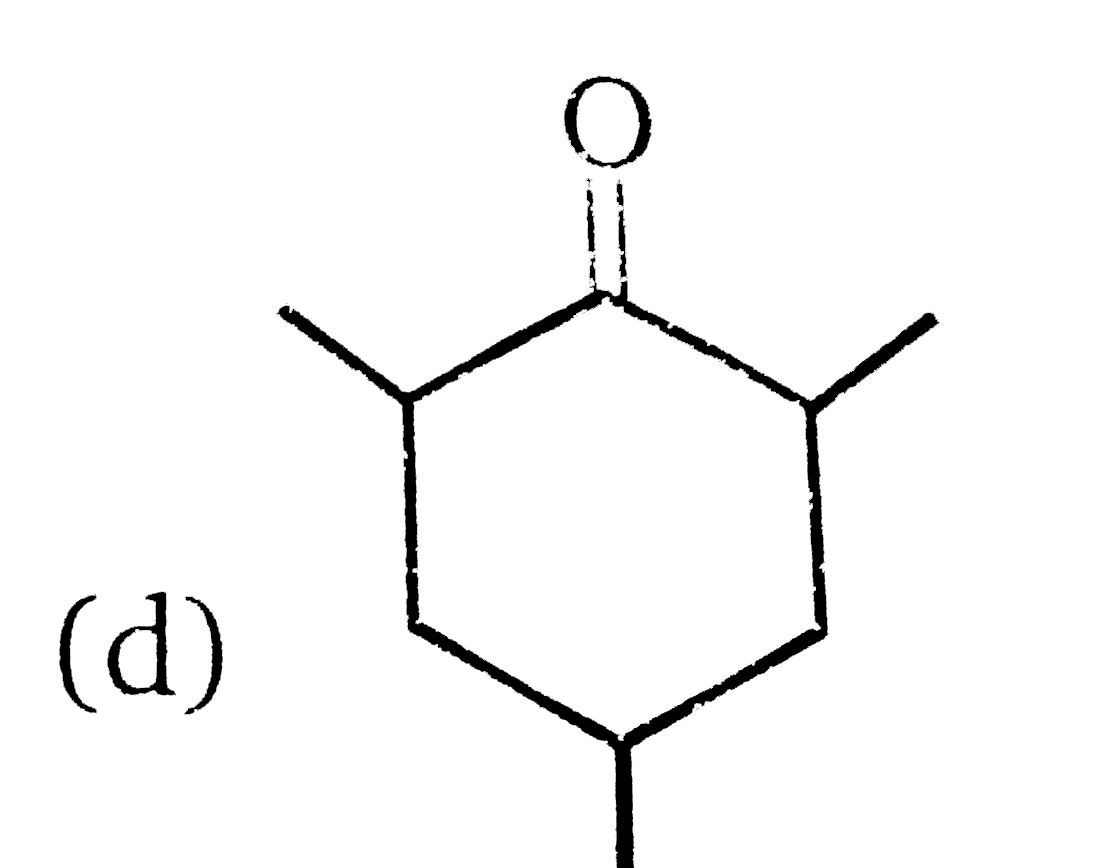
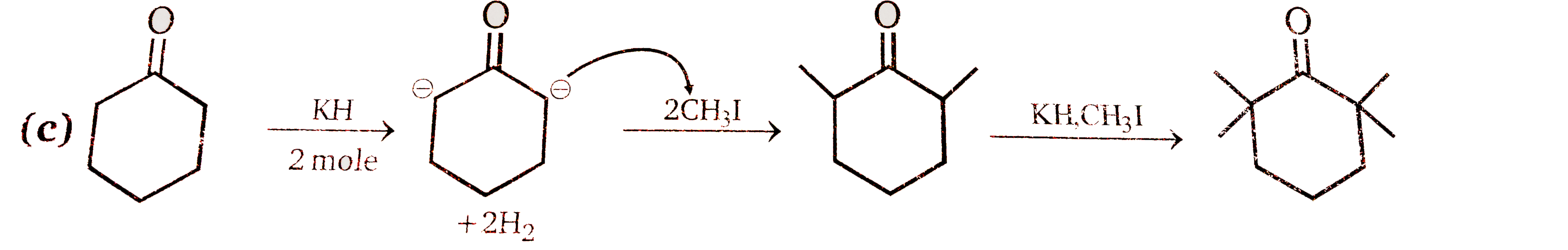
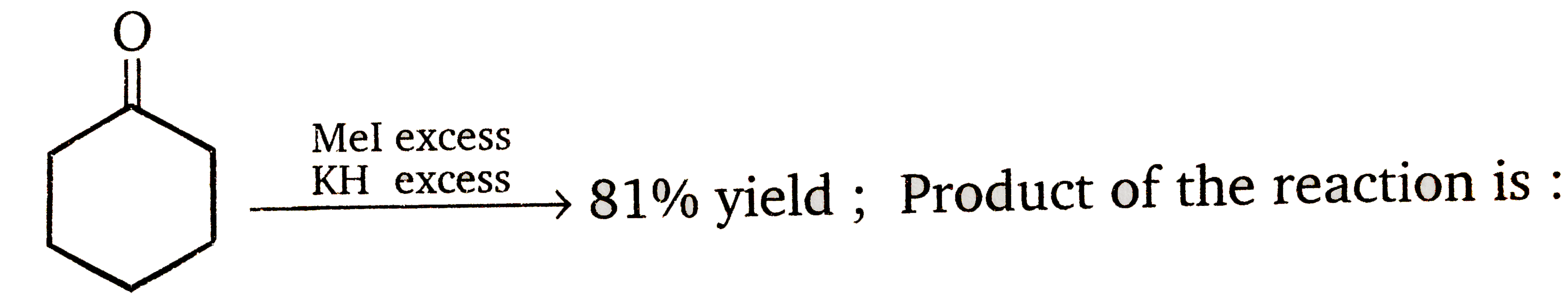A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES AND KETONES
MS CHOUHAN|Exercise Level 1 (Q.121 To Q.150)|1 VideosALDEHYDES AND KETONES
MS CHOUHAN|Exercise Level 2|7 VideosALCOHOLS FROM CARBONYL COMPOUNDS
MS CHOUHAN|Exercise Additional Objective Questions (Multiple Correct Choice Type )|1 VideosALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO THE CARBONYL GROUP
MS CHOUHAN|Exercise ADDITIONAL OBJECTIVE QUESTIONS (SINGLE CORRECT CHOICE TYPE)|17 Videos
Similar Questions
Explore conceptually related problems
MS CHOUHAN-ALDEHYDES AND KETONES -Level 2
- 81% yield , Product of the reaction is :
Text Solution
|
- Consider the possible formation of an aldehyde or ketone product when ...
Text Solution
|
- Wittig reaction : The reaction of a phosphorus ylide with an aldehyd...
Text Solution
|
- Wittig reaction : The reaction of a phosphorus ylide with an aldehyd...
Text Solution
|
- Wittig reaction : The reaction of a phosphorus ylide with an aldehyd...
Text Solution
|
- Wittig reaction : The reaction of a phosphorus ylide with an aldehyd...
Text Solution
|
- Wittig reaction : The reaction of a phosphorus ylide with an aldehyd...
Text Solution
|
- (A)overset(HgSO(4))underset("dil." H(2)SO(4))(to) (B) underset((2) H^(...
Text Solution
|