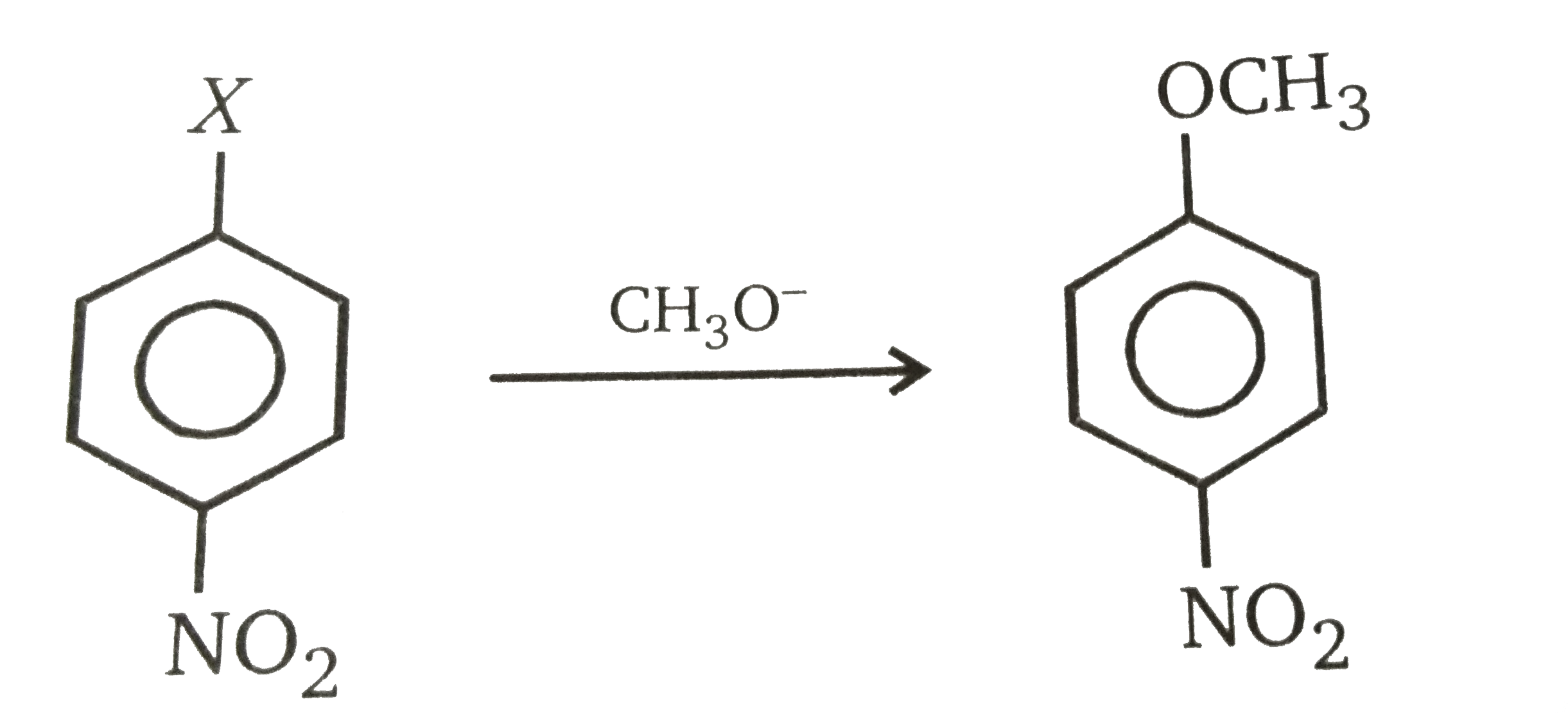
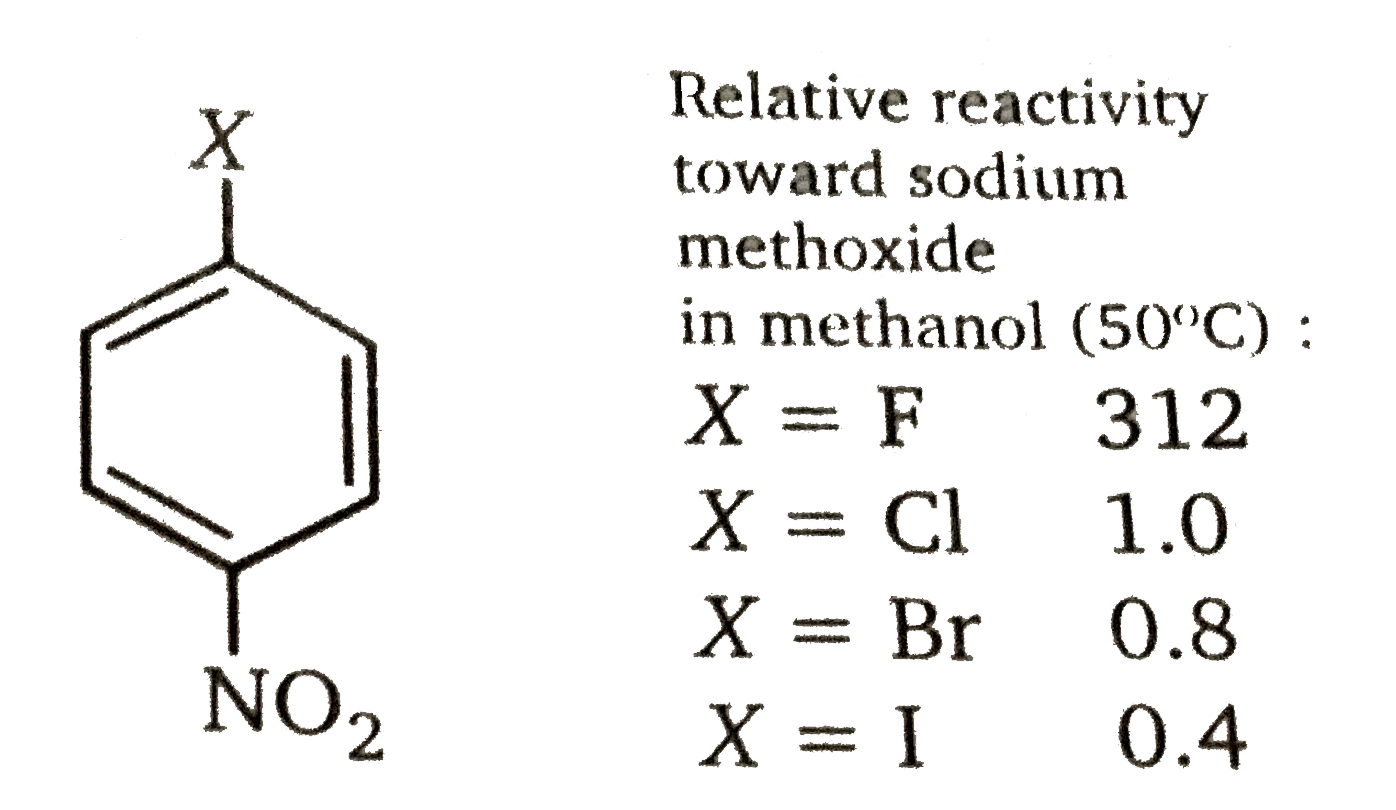Fluoride is the most reactive leaving group in nucleophilic aromatic substitution, iodide the least reactive.
Kinetic studies of these reactions reveal that they follow a second-order rate law:
Rate `= k`(Aryl halide][Nucleophile]
Second -order kinetics is usually interpreted in terms of a bimolecular rate-determing step. In this case, then we look for a mechanism in which both the aryl halide and the nucleophile are involved in the slowest step. Such a mechanism is described in the following section
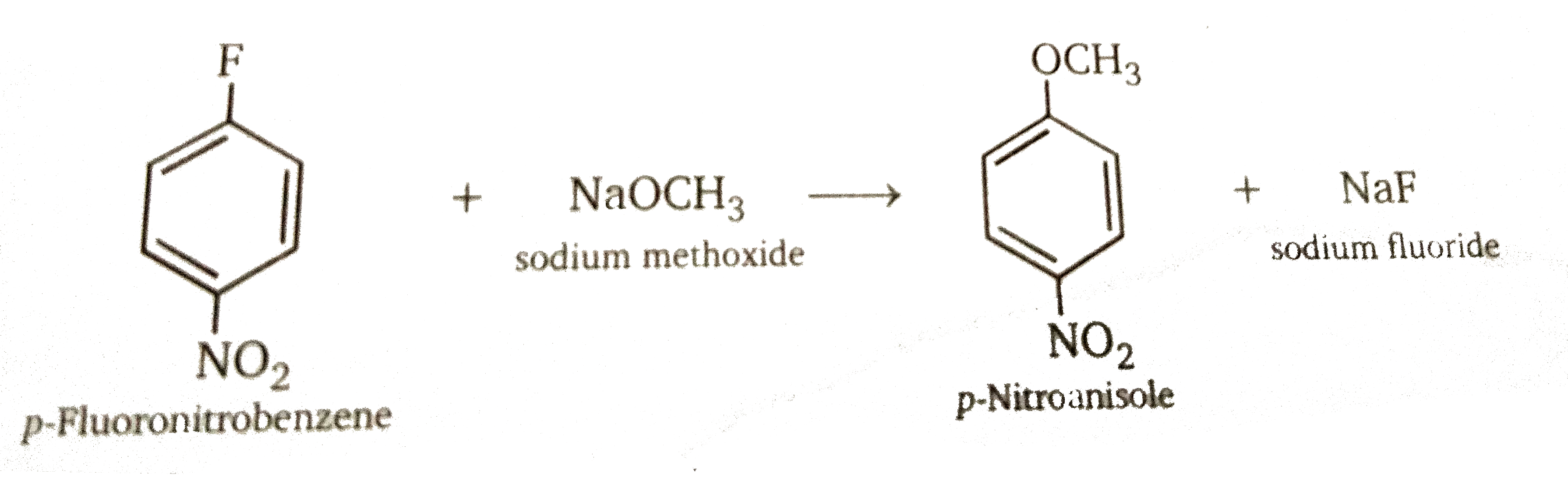
Step 1: Addition stage. the nucleophile, in this case methoxide ion, adds to the carbon atom that bears the leaving group to give a cyclohexadienyl anion intermediate.
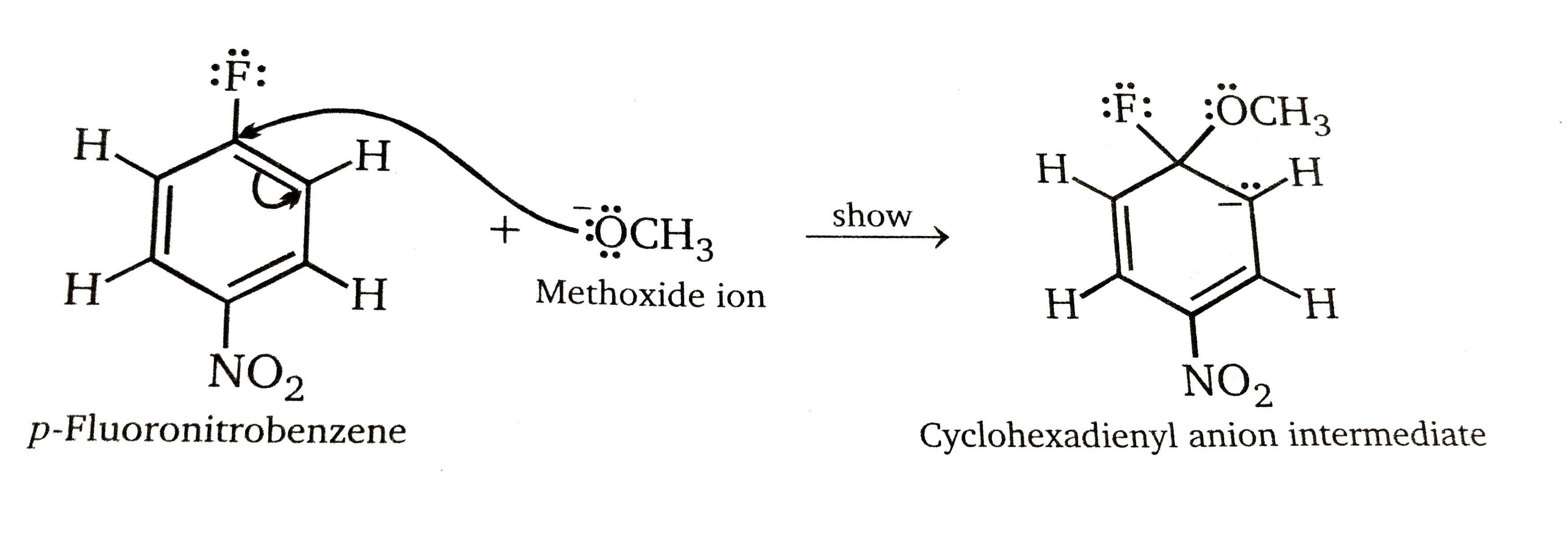
Step 2: Elimination stage. Loss of halide from the cyclohexadienyl intermediate restores the aromaticity of the ring and gives the product of nucleophilic aromatic substitution.
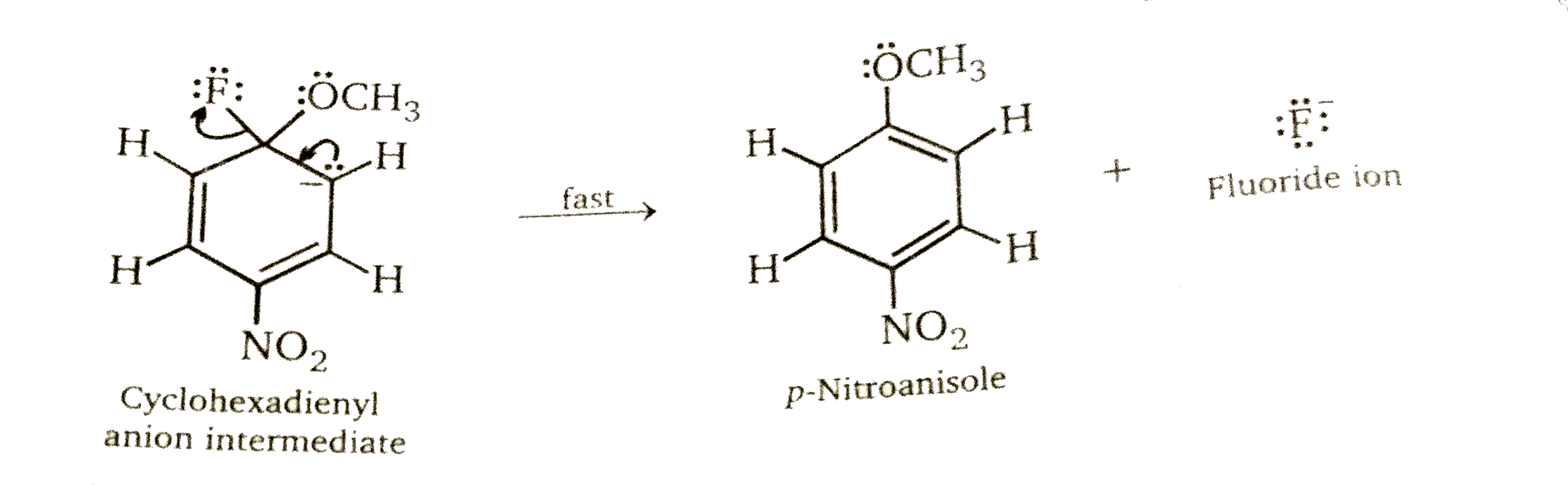
Because aryl flouorides have the strongest carbon-halogen bond and react fastest, the rate-determining step cannot involve carbon-halogen bond cleavage. According to the mechanism in fig. the carbon-halogen bond breaks in the rapid elimination step that follows the rate-determining addition step. The unusually high reactivity of aryl fluorides arises because fluorine is the most electronegative of the halogens, and its greater ability to attract electrons increase the rate of formation of the cyclohexadenyl anion intermediate in the first step of the mechanism.

Before leaving this mechanistic discussion, we should mention that the addition-elimination mechanism for nucleophilic aromatic substitution illustrates a principle worth remembering. The words activating and deactivating as applied to substituent effects in organic chemistry are without meaning when they stand alone. When we say that group is activating or deactivating, we need to specify the reation type that is being considered. A nitro group is a strongely deactivating substituent in electrophilic aromatic substitution, where it markedly destabilizes the key cyclohexadienyl cation intermediate.