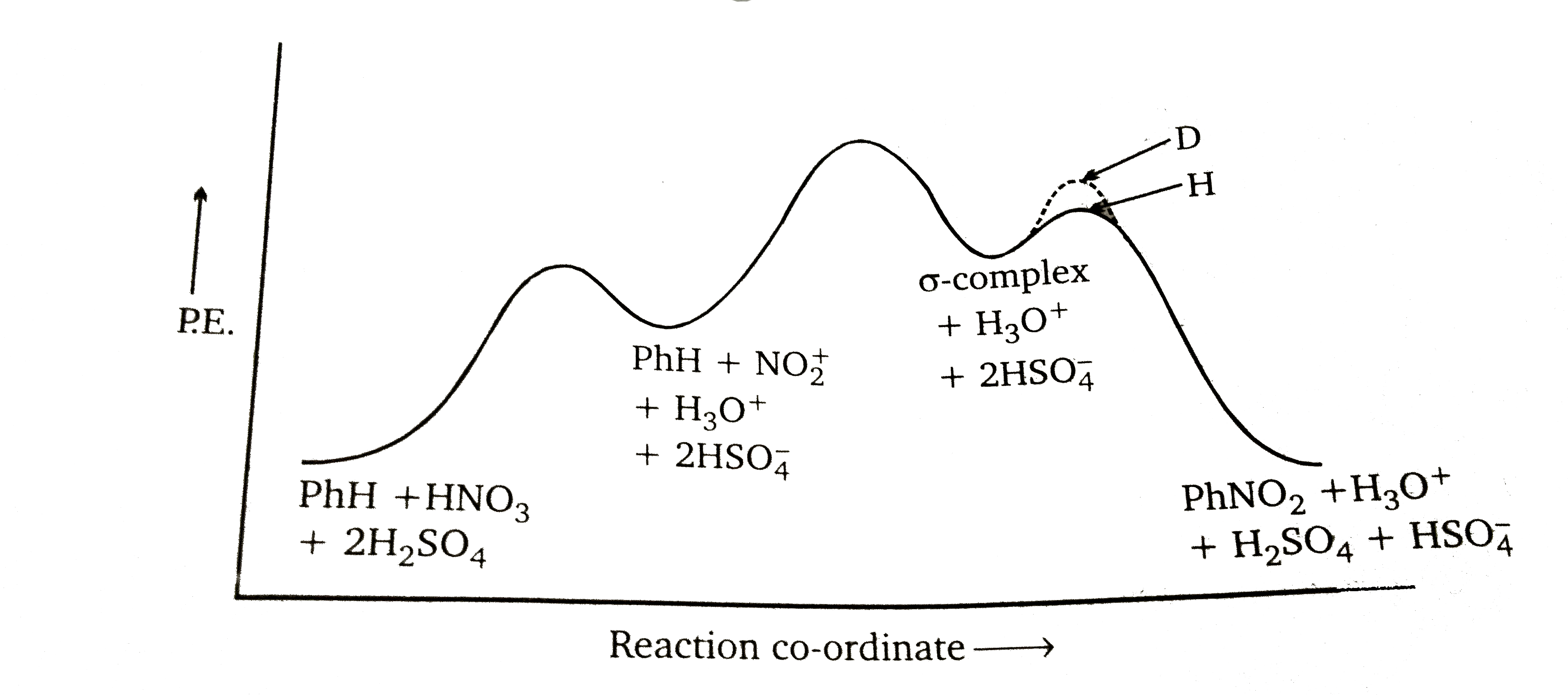A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
MS CHOUHAN|Exercise Level 1 (Q.31 To Q.60)|1 VideosAROMATIC COMPOUNDS
MS CHOUHAN|Exercise Level 1 (Q.121 To Q.150)|3 VideosAN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASES
MS CHOUHAN|Exercise ADDITIONAL QUESTION (SINGLE CORRECT CHOICE TYPE )|32 VideosBIOMOLECULES
MS CHOUHAN|Exercise Level 2|1 Videos
Similar Questions
Explore conceptually related problems