Text Solution
Verified by Experts
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS:|49 VideosSOME BASIC CONCEPTS OF CHEMISTRY
SUBHASH PUBLICATION|Exercise TWO MARKS QUESTIONS AND ANSWERS|38 VideosS-Block Elements
SUBHASH PUBLICATION|Exercise Three marks questions and answers|8 VideosSTATES OF MATTER : GASES AND LIQUIDS
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS|25 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-SOME BASIC CONCEPTS OF CHEMISTRY -THREE MARKS QUESTION WITH ANSWERS
- Calculate the moles of NaOH required to neutralize the solution produc...
Text Solution
|
- (a) A sample of NaOH weighting 0.38 g is dissolved in water and the so...
Text Solution
|
- Calculate the amount of water (g) produced by the combustion of 16 g o...
Text Solution
|
- Explain the classification of matter
Text Solution
|
- (a) How many significant figures are there in 1.00 xx 10^(4)? (b) On...
Text Solution
|
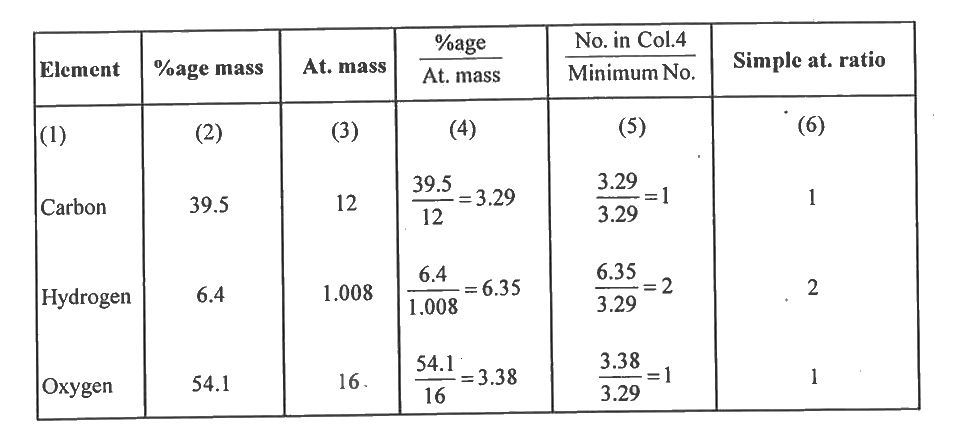
- An organic monobasic acid was found to contain 39.5% carbon, 6.4% hydr...
Text Solution
|
- Calcualte the mass of 95% pure MnO(2) to produce 35.5 g of Cl(2) as pe...
Text Solution
|
- Classify the following into homogenous and heterogenous mixtures: milk...
Text Solution
|
- Discuss the properties of matter.
Text Solution
|
- Express the following in scientific notation: (a) 4007 (b) 0.0068 ( c)...
Text Solution
|
- Express the following S.I. bases units using power 10 notations (a) 48...
Text Solution
|
- Calculate the weight of CaO that can be obtained by heating 200kg of l...
Text Solution
|
- 20g of sample containing Ba(OH)(2) is dissolved in 10 ml of 0.5 M HCl...
Text Solution
|
- Express the following in S.I. units (a) 100 miles per hours, (b) 5 fee...
Text Solution
|
- Explain law of multiple proportions with example:
Text Solution
|
- Write the postulates of Daltons Atomic theory.
Text Solution
|
- Define formula mass and given an example.
Text Solution
|
- Write the empirical formula of the compounds having molecular formulae...
Text Solution
|