Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROGEN
SUBHASH PUBLICATION|Exercise Three marks questions and answers|15 VideosHYDROGEN
SUBHASH PUBLICATION|Exercise Three marks questions and answers|15 VideosHYDROCARBONS
SUBHASH PUBLICATION|Exercise THREE MARKS QUESTIONS AND ANSWERS|25 VideosP-BLOCK ELEMENTS
SUBHASH PUBLICATION|Exercise Three mark questions and answers|24 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-HYDROGEN-Two marks questions and answers
- How to prepare hydrogen peroxide from BaO(2)?
Text Solution
|
- Dilute solution of hydrogen peroxide cannot be heated strongly for its...
Text Solution
|
- Can sodium bicarbonate make water hard ?
Text Solution
|
- Which of the hydrogen or deuterium undergoes reactions more rapidly an...
Text Solution
|
- Can we remove completely temporary hardness due to Mg(HCO(3))(2) by bo...
Text Solution
|
- H(2)O(2) acts as on oxidizing agent as well as reducing agent Why ?
Text Solution
|
- Why do lakes freeze from top towards bottom ?
Text Solution
|
- A mixture of H(2)O(2) and hydrzine with copper (II) is used as a rocke...
Text Solution
|
- Water cannot be used to extinguish petrol fires. Why ?
Text Solution
|
- What is the volume strenth of H(2)O(2)?
Text Solution
|
- Hard water is softened before using in boilers. Expain.
Text Solution
|
- What are the uses of Heavy Water ?
Text Solution
|
- Show the oxidising property of H(2)O(2) with PbS.
Text Solution
|
- Discuss the position of hydrogen in the periodic table is not justifie...
Text Solution
|
- Explain property of H(2)O(2)"with"MnO(4)^(-) in acidic medium.
Text Solution
|
- How does H(2)O(2) reduces iodine in reducing property ?
Text Solution
|
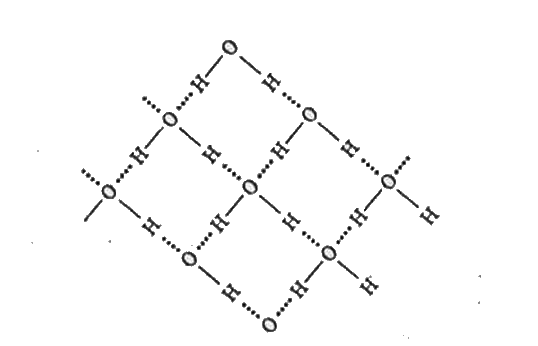
- Describe the structure of common from of ice.
Text Solution
|
- Distinguish clearly between (a) hard and soft water, (b) temporary har...
Text Solution
|
- What is the structure of H(2)O(2)? Draw a shematic diagram indicating ...
Text Solution
|
- Sea water can't be used in boiler. Explain given chemical equations.
Text Solution
|