Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-HYDROCARBONS -THREE MARKS QUESTIONS AND ANSWERS
- How to prepare alkene by hydrogenation of 2-butyne?
Text Solution
|
- Explain dehydrohalogenation of a alkyl halide.
Text Solution
|
- Discuss the structural elucidation of benzene.
Text Solution
|
- Explain the mechanism of halogenation or chlorination of benzene.
Text Solution
|
- Explain the mechanism of nitration of benzene .
Text Solution
|
- Explain the mechanism of sulphonation of benzene?
Text Solution
|
- Explain the mechanism of Friedel craft alkylation of benzene.
Text Solution
|
- Explain Saytzeff's rule with an example.
Text Solution
|
- Explain peroxide effect.
Text Solution
|
- What happens when water is added to ethene and propene?
Text Solution
|
- Discuss isomerism in alkynes.
Text Solution
|
- Explain the structure of triple bond of ethynes.
Text Solution
|
- Explain resonance and stability of benzene.
Text Solution
|
- What are the conditions for a molecule to aromatic?
Text Solution
|
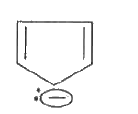
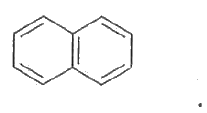
- Using IIuckel's rule, show that cyclopentadienyl anion and naphthalene...
Text Solution
|
- Write Friedel Craft acylation of benzene using CH(3)COCI and (CH(3) CO...
Text Solution
|
- Explain the mechanism of Friedel craft's acylation reaction.
Text Solution
|
- Explain ortho and para directing group influencing in benzene.
Text Solution
|
- Explain meta direacting groups influencing in benzene.
Text Solution
|
- Compare stability and dihedral angle of ethane using sawhorse and newm...
Text Solution
|