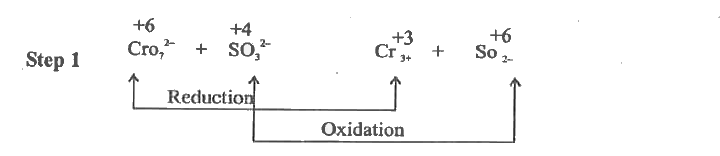Text Solution
Verified by Experts
Topper's Solved these Questions
ANNUAL EXAMINATION QUESTION PAPER NORTH-2017
SUBHASH PUBLICATION|Exercise PART-D|19 VideosANNUAL EXAMINATION QUESTION PAPER NORTH-2017
SUBHASH PUBLICATION|Exercise PART-E|10 VideosANNUAL EXAMINATION QUESTION PAPER NORTH-2017
SUBHASH PUBLICATION|Exercise PART-B|10 VideosANNUAL EXAMINATION QUESTION PAPER NORTH-2018
SUBHASH PUBLICATION|Exercise PART-E|8 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNUAL EXAMINATION QUESTION PAPER NORTH-2017-PART-C
- What are isoelectronic species?
Text Solution
|
- How electronegativity vary down the group in the periodic table?
Text Solution
|
- Explain the formation of BCl(3) molecule based on hybridisation.
Text Solution
|
- Write any three postulates of VSEPR theory.
Text Solution
|
- Define dipolement of a polar bond. Show that BeF(2) molecule has zero ...
Text Solution
|
- Balance the following redox reaction by using Oxidation number method ...
Text Solution
|
- Complete the following reactions (i)C+H(2)Ooverset(1270K)(to) (ii) P...
Text Solution
|
- Write chemical formula for the following: a. Washing soda b. plaster...
Text Solution
|
- Name a member of group 14 element in the periodic table which is used ...
Text Solution
|
- Give reason : (i)Boron is used as control rods in nuclear reactor. (...
Text Solution
|