Text Solution
Verified by Experts
Topper's Solved these Questions
ANNUAL EXAMINATION QUESTION PAPER NORTH-2018
SUBHASH PUBLICATION|Exercise PART-E|8 VideosANNUAL EXAMINATION QUESTION PAPER NORTH-2018
SUBHASH PUBLICATION|Exercise PART-C|11 VideosANNUAL EXAMINATION QUESTION PAPER NORTH-2017
SUBHASH PUBLICATION|Exercise PART-E|10 VideosANNUAL EXAMINATION QUESTION PAPER NORTH-2019
SUBHASH PUBLICATION|Exercise PART-D|27 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNUAL EXAMINATION QUESTION PAPER NORTH-2018-PART-D
- Write the postulates of Daltons Atomic theory.
Text Solution
|
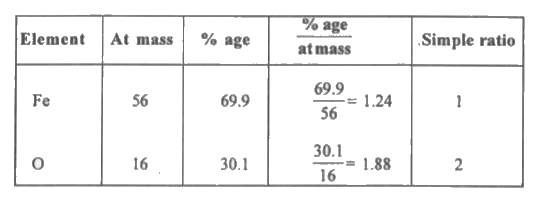
- Determine the empirical formula of an oxide of Iron which has 69.9% Ir...
Text Solution
|
- Explain the significance of Principal, Azimuthal and magnetic quantum ...
Text Solution
|
- Calculate the frequency of yellow radiation having wavelength 5800Å. ...
Text Solution
|
- State Pauli's exclusion principle. Give the possible values of l for n...
Text Solution
|
- Write De Broglie equation and explain the terms involved in it.
Text Solution
|
- Write any three postulates of Kinetic molecular theory of gases.
Text Solution
|
- Define (i) Boyle temperature (ii) Critical Volume (V(C))
Text Solution
|
- The enthalpy of combustion of one mole of benzene,carbon and hydrogen ...
Text Solution
|
- Define Entropy. What is the value of Entropy change at equilibrium in ...
Text Solution
|
- State Hess's law of constant heat summation.
Text Solution
|
- Explain an example for a extensive property.
Text Solution
|
- Explain Born-Haber cycle for the fomation of 1 mole of sodium chloride...
Text Solution
|
- State Le-Chatelier's principle.
Text Solution
|
- Explain the effect of temperature and pressure on the equilibrium equa...
Text Solution
|
- What is the buffer solution? Give an example.
Text Solution
|
- Explain Lewis acid base concept of acid and base.
Text Solution
|
- Define common ion effect. Mention any one factor, which affect acid st...
Text Solution
|
- Give the value of ionic product of pure water at 298 K.
Text Solution
|