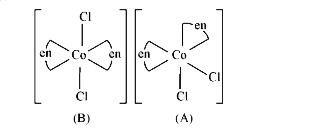
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A reaction of cobalt (III) chloride and ethylenediamine is a 1 : 2 mol...
Text Solution
|
- Neither optical nor geometrical isomers can be distinguished by mass s...
Text Solution
|
- underset("hv") overset(Cl(2))to Products Find out no of correct stat...
Text Solution
|
- STATEMENT-1: (A) is optically active and (B) is optically inactive. ...
Text Solution
|
- Optically active isomer (A) of (C(5)H(9)Cl) on treatment with one mole...
Text Solution
|
- A reaction of cobalt (III) chloride and ethylenediamine is a 1 : 2 mol...
Text Solution
|
- Which isomer of [CoCl(2)(en)(2)]^(+) does not show optical isomerism ?
Text Solution
|
- An optically active compound A (C(10)H(14)) gets oxidised to benzoic a...
Text Solution
|
- Isomerism| types of isomers|geometrical isomers|optical isomers
Text Solution
|