Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Total number of lone pair of electrons in XeOF4 is :
Text Solution
|
- Total number of lone pair of electrons in XeOF4 is :
Text Solution
|
- The total number of lone-pairs of electrons in melamine is.
Text Solution
|
- The total number of lone pairs of electrons in melamine is:
Text Solution
|
- Total number of lone pairs of electron in melamine is
Text Solution
|
- Total number of lone pair of electrons is XeOF4 is
Text Solution
|
- The total number of lone pairs of electrons in melamine
Text Solution
|
- Number of lone pair and bond pairs present on Xe of XeOF4 molecule is
Text Solution
|
- Total number of lone pair of electrons in XeOF4 IS:
Text Solution
|
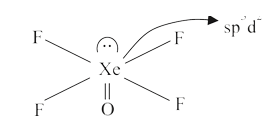 Number of lone pairs = 1.
Number of lone pairs = 1.