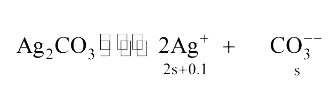Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If K(sp) of Ag(2)CO(3) is 8 xx 10^(-12), the molar solubility of Ag(2)...
Text Solution
|
- Solid AgNO(3) is gradually added to a solution which is 0.01M n Cl^(-)...
Text Solution
|
- Solid AgNO(3) is gradually added to a solution which is 0.01M n Cl^(-)...
Text Solution
|
- The molar solubility of AgCl in 1.8 M AgNO(3) solution is (K(sp) of Ag...
Text Solution
|
- Solid AgNO(3) is added to a solution which 0.1 M in Cl^(-) and 0.1 M i...
Text Solution
|
- At 30^(@)C , the solubility of Ag(2)CO(3)(K(sp) = 8 xx 10^(-12)) would...
Text Solution
|
- The K(sp) of Ag(2)CrO(4) is 1.1xx10^(-12) at 298K. The solubility (in...
Text Solution
|
- 298 K पर Ag(2)CrO(4) के K(sp) का मान 1.1 xx 10^(-12) है| 0.1 M AgNO(3)...
Text Solution
|
- If K(sp) of Ag(2)CO(3) is 8 xx 10^(-12), the molar solubility of Ag(2)...
Text Solution
|