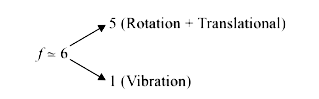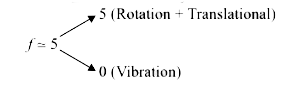A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The specific heats, C(P) and C(V) of a gas of diatomic molecules, A ar...
Text Solution
|
- The specific heats, C(P) and C(V) of a gas of diatomic molecules, A ar...
Text Solution
|
- The ratio C(P)//C(V) represented by gamma correspends ot the atomicity...
Text Solution
|
- Consider, two ideal diatomic gases A and B at some temperature T. Mole...
Text Solution
|
- Consider two diatomic ideal gases A &B at some temperature T. Molecule...
Text Solution
|
- A diatomic gas molecule has translational, rotational and vibrational ...
Text Solution
|
- एक द्विपरमाणु गैस A के अणुओं की विशिष्ट ऊष्मायें (Jmol^(-1)K^(-1) की इ...
Text Solution
|
- C(v) = 5/2 R, for 1 mol of any diatomic ideal gas. The value of the ra...
Text Solution
|
- Specific heat at constant pressure C(p) for a diatomic gas at N.T.P. i...
Text Solution
|