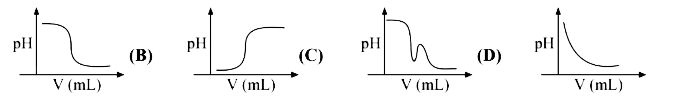A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In an acid base titration, 0.1 M HCI solution was added to the NaOH so...
Text Solution
|
- Which of the following solution can be titrated with HCI as well as Na...
Text Solution
|
- A solution of weak acid was titrated with base NaOH. The equivalence p...
Text Solution
|
- Acetic acid is titrated with NaOH solution. Which of the following sta...
Text Solution
|
- In the titration of a weak acid of known concentratin with a standard ...
Text Solution
|
- A 100 ml solution of 0.1 N HCl was titrated with 0.2? N NaOH solution....
Text Solution
|
- In an acid base titration, 0.1 M HCI solution was added to the NaOH so...
Text Solution
|
- When a 40 mL of a 0.1 M weak base in titrated with 0.16 M HCl, the pH ...
Text Solution
|
- Calculate the pH at the equivalence point when a solution of 0.1 M ace...
Text Solution
|