A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
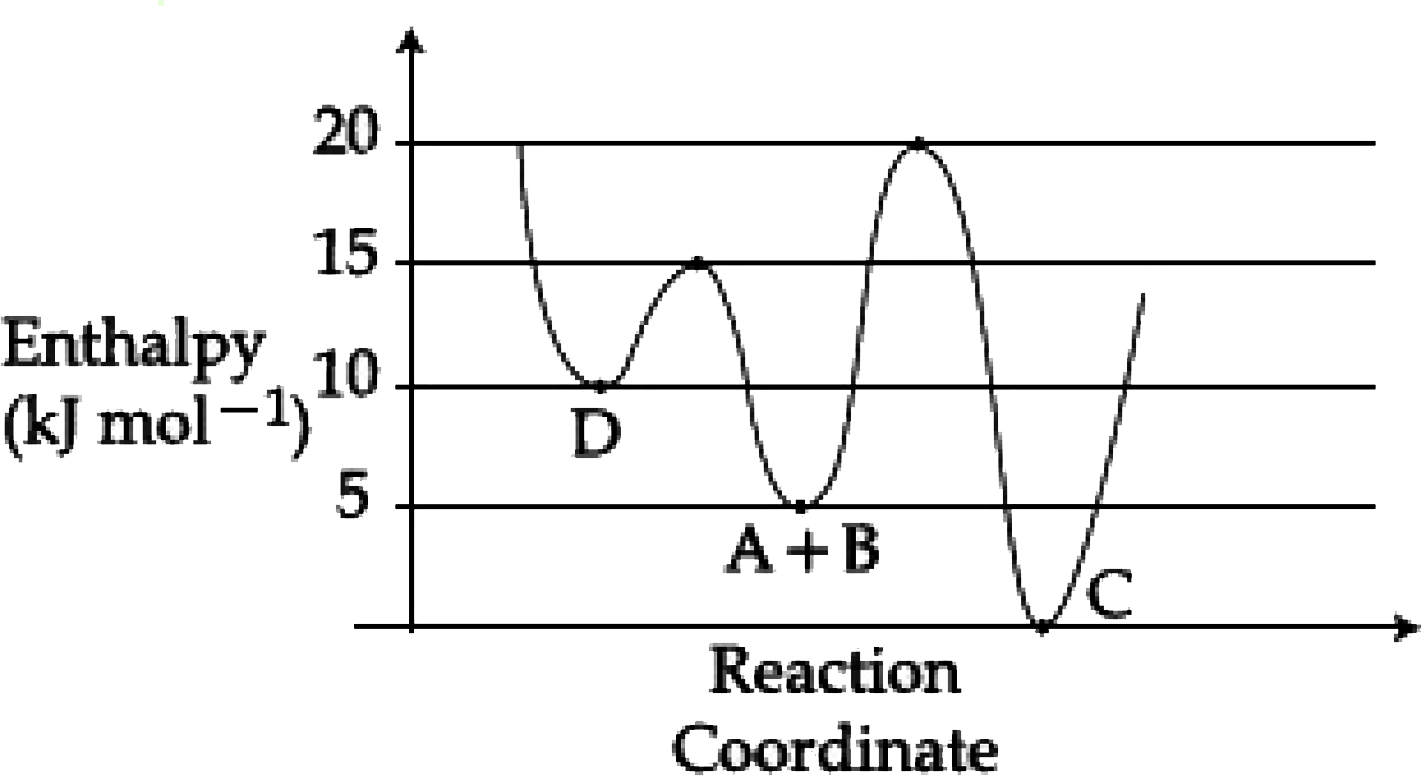
- Consider the given plot of enthalpy of the following reaction between ...
Text Solution
|
- Consider the given plot of enthalpy of the following reaction between ...
Text Solution
|
- Consider the following diagram. Identify A, B, C and D
Text Solution
|
- Consider the following sequence of reactions identify A, B, C and...
Text Solution
|
- Given the following diagram for the reaction A+B rarr C+D The enthalpy...
Text Solution
|
- Consider the following sequence of reactions- Identify A, B, C an...
Text Solution
|
- Consider the following figure and identify the A,B,C and D:-
Text Solution
|
- निम्नलिखित A एवं B के बीच अभिक्रिया की एन्थैल्पी के दिये गये प्लॉट पर ...
Text Solution
|
- Given a+b+c+d = 0 which of the following statement is incorrect
Text Solution
|