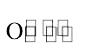A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The pH of 0.02MNH(4)Cl solution will be : [Given K(b) (NH(4)OH) = 10^...
Text Solution
|
- Calculate the degree of hydrolysis and pH of 0.2M solution of NH(4)C1 ...
Text Solution
|
- Calculate pH of a buffer solution that contains 0.1M NH(4)OH(K(b)=10^(...
Text Solution
|
- Concentration of NH(4)Cl and NH(4)OH in a buffer solution are in the r...
Text Solution
|
- A buffer solution with pH 9 is to be prepared by mixing NH(4)OH soluti...
Text Solution
|
- Calculate the pH of a solution obtained by mixing 5 mL of 0.1 M NH(4) ...
Text Solution
|
- 30 मिली 0.2 M NH(4)Cl विलयन में 0.3 M NH(4)OH के कितने मिली मिलाने पर ...
Text Solution
|
- The pH of 0.02MNH(4)Cl solution will be : [Given K(b) (NH(4)OH) = 10^...
Text Solution
|
- Calculate pH of the buffer solution containing 0.15 mole of NH(4)OH " ...
Text Solution
|