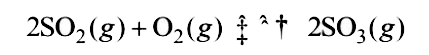A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reaction, 2SO2(g) +O2->2SO3(g) DeltaH = -57.2 kJ mol^(-1) and ...
Text Solution
|
- For the reaction, 2SO2(g) +O2->2SO3(g) DeltaH = -57.2 kJ mol^(-1) and ...
Text Solution
|
- The equilibrium constant for the reaction, SO3(g)hArr SO2(g)+1/2O2(g...
Text Solution
|
- The rate of disappearance of SO(2) in the reaction 2SO(2) + O(2) rarr ...
Text Solution
|
- The standard Gibbs energy change for the reaction 2SO2(g) +O2(g) to ...
Text Solution
|
- যদি 2SO2 (g) + O2(g) hArr 2SO3 (g) বিক্রিয়ার ক্ষেত্রে সাম্যধ্রুবক এর ...
Text Solution
|
- अभिक्रिया , 2SO(2)(g) + O2(g) hArr 2SO3(g) के लिए DeltaH=-57.2 "kJ mol...
Text Solution
|
- अभिक्रिया , 2SO(2)(g) + O2(g) hArr 2SO3(g) के लिए DeltaH=-57.2 "kJ mol...
Text Solution
|
- Kp for the reaction 2SO3(g) +O2(g) leftrightarrow 2SO3(g) is 2.5 times...
Text Solution
|