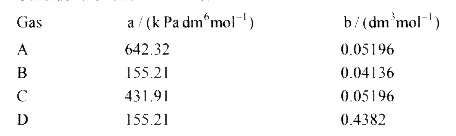A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following table: a and b are van de Waals constants....
Text Solution
|
- If two gases have the same value of b but different values of a ( a an...
Text Solution
|
- The van der Waals constant for gases A, B, and C are as follows A...
Text Solution
|
- The van der Waals constant for gases A , B , and C are as follows Answ...
Text Solution
|
- Arrange the van der Waals constant for the gases .
Text Solution
|
- van der Waals constant b in corrected equation for real gases represen...
Text Solution
|
- Consider the following table: a and b are van de Waals constants....
Text Solution
|
- निम्न तालिका पर विचार कीजिए a तथा b वन्डरवाल्स स्थिरांक है | गै...
Text Solution
|
- van der Waals constant b in corrected equation for real gases represen...
Text Solution
|