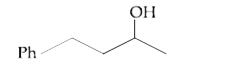
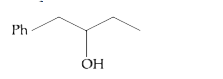
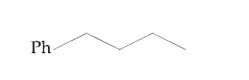
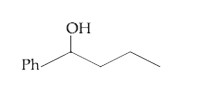
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Heating of 2-chloro-1-phenylbutane with EtOK/EtOH gives X as the major...
Text Solution
|
- 2-Chloro-1-phenylbutane underset(EtOH)overset(NaOH)tounderset(NaBH(4))...
Text Solution
|
- Give the major product the following reaction major product
Text Solution
|
- 2-Chloro-2-methylpropane on reaction with aqueous KOH gives X as the m...
Text Solution
|
- 2-Chloro-2-methylpropane on reaction with NH(3) gives major product
Text Solution
|
- Treatment of benzene with CO/HCl in the presence of anhydrous "AlCl"(3...
Text Solution
|
- Treatment of benzene with CO/HCl in the presence of anhydrous "AlCl"(3...
Text Solution
|
- Heating of 2-chloro-1-phenylbutane with EtOK/EtOH gives X as the major...
Text Solution
|
- Heating of 2-chloro-l-phenylbutane with EtOK // EtOH gives X as the ma...
Text Solution
|