A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
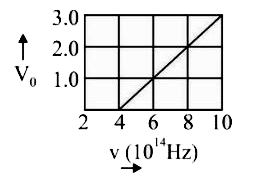
- The stopping potential V(0) (in volt) as a function of frequency (v) ...
Text Solution
|
- In an experiment on photoelectric effect the frequency f of the incide...
Text Solution
|
- The figure shows different graphs between stopping potential (V(0)) an...
Text Solution
|
- For photoelectric effect in sodium, fig. shows the plot of cut-off vol...
Text Solution
|
- The stopping potential (V(0)) versus frequency (v) plot of a substance...
Text Solution
|
- सोडियम धातु के लिए थ्रेशोल्ड तरंगदैर्घ्य 680nm है। इसके लिए कार्य-फलन ...
Text Solution
|
- The stopping potential V(0) (in volt) as a function of frequency (v) f...
Text Solution
|
- The stopping potential vs frequency plot for a substance is shown in t...
Text Solution
|
- यहाँ आरेख में एक सोडियम-उत्सर्जक के लिए आवृत्ति (v) के फलन के रूप में ...
Text Solution
|