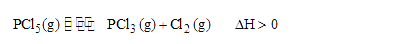A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The gas phase reaction PCl(5)(g)<=> PCl(3)(g)+Cl(2)(g) is an endother...
Text Solution
|
- For the reaction, PCl(3)(g)+Cl(2)(g) hArr PCl(5)(g) , the position of ...
Text Solution
|
- For the reaction PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) the forward reactio...
Text Solution
|
- For the reaction, PCl(5)(g)to PCl(3)(g)+Cl(2)(g), The forward reaction...
Text Solution
|
- अभिक्रिया, PCl(5)(g) ltimplies PCl(3)(g) + Cl(2)(g) के लिए साम्य ताप प...
Text Solution
|
- For the reaction, PCl(5)(g)to PCl(3)(g)+Cl(2)(g)
Text Solution
|
- The gas phase reaction PCl(5)(g) PCl(3)(g)+Cl(2)(g) is an endothermic ...
Text Solution
|
- In the reaction PCl(5)(g) rarr PCl(3)(g) + Cl(2)(g) PCl(5),PCl(3)"and"...
Text Solution
|
- At a given temperature the equilibrium constant for the reaction PCl(5...
Text Solution
|