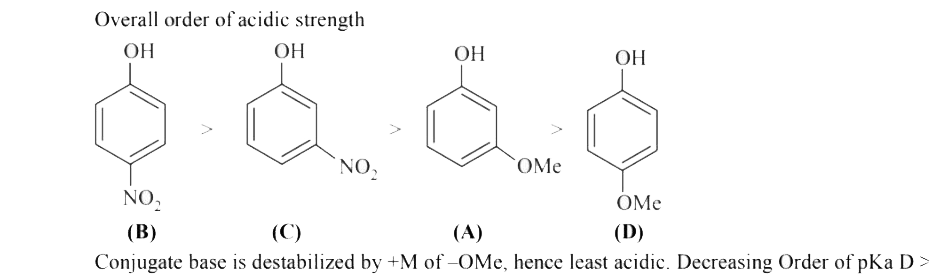
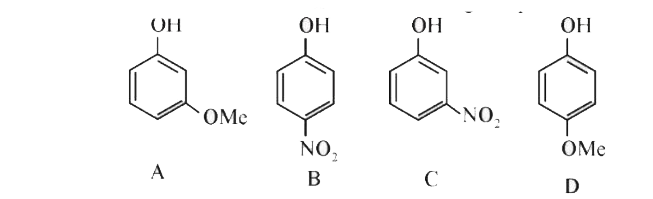
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The decreasing order of the pKa values of the following compounds is:
Text Solution
|
- The decreasing order of pKa value of the following is : (I) CH-=CH (II...
Text Solution
|
- The pKa value of (I) is more than the pKa value to (II). Nonaromatic c...
Text Solution
|
- Correct Decreasing order of pK(b) value of Following Compound is
Text Solution
|
- Which of the following represents the correct order of increasing pKa ...
Text Solution
|
- Match the pKa values with the given compounds
Text Solution
|
- The decreasing order of the pKa values of the following compounds is:
Text Solution
|
- The increasing order of the pKa values of the following compounds is
Text Solution
|
- Which of the following compounds would have the smallest value for PKa...
Text Solution
|
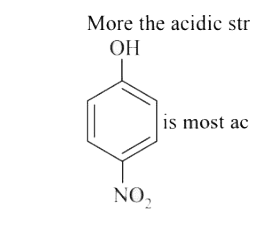 is most acidic as it’s conjugate base is resonance stabilized by `NO_2` group Overall order of acidic strength
is most acidic as it’s conjugate base is resonance stabilized by `NO_2` group Overall order of acidic strength