Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
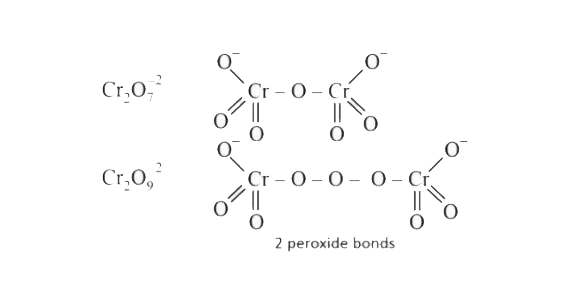
- Number of peroxide bonds in the compound Cr2 O(9)^(2-) .
Text Solution
|
- The maximum number of identical Cr-O bond lengths in Cr2 O7^(2-) would...
Text Solution
|
- Total number of covalent bonds in C(3)O(2) is The total number of sigm...
Text Solution
|
- Hydrogen peroxide on reaction with sulphurated K(2)Cr(2)O(7) in ethera...
Text Solution
|
- The number of pi -bonds in the following compound O(2)N-C-=C-NO(2) is:
Text Solution
|
- Number of S-O-S bonds in S(3)O(9) is
Text Solution
|
- Calculate the number of moles in 17g of hydrogen peroxide (H(2)O(2)) .
Text Solution
|
- Which of the following compounds does not have peroxide bond?
Text Solution
|
- Number of peroxide bonds in the compound Cr2 O(9)^(2-) .
Text Solution
|