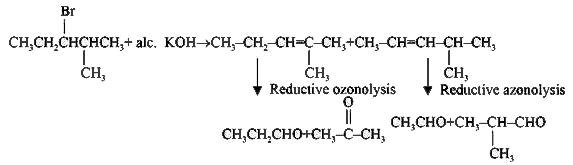
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A halide with formula C(6)H(13)Br gave two isomeric alkenes A and B wi...
Text Solution
|
- Correct order of reactivity of CH(3) CHO, C(2) H(3) COCH(3) and CH(3) ...
Text Solution
|
- (a) Write the reagent used in the following : C(6)H(5)COCH(3)overset...
Text Solution
|
- A halide with formula C(6)H(13)Br gave two isomeric alkenes A and B wi...
Text Solution
|
- Amongest the following maximum number of compounds which give iodofor...
Text Solution
|
- Compounds showing Cannizaro' s reaction are A) CH(3)CH(2)CHO ( ...
Text Solution
|
- An alkyl halide with molcular formula C(6)H(13)Br on dehydrohalogenati...
Text Solution
|
- An organic halide with formula C(6)H(13)Br on heating with alc. KOH gi...
Text Solution
|
- How can we distinguish chemically the following pairs of compounds? ...
Text Solution
|