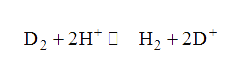Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A solution containing H^(+) and D^(+) ions is in equilibrium with a mi...
Text Solution
|
- At 25^(@)C and 1 atm pressure, the partial pressure in equilibrium mix...
Text Solution
|
- For the given cell Pt(D(2)|D^(o+))||H^(o+)|Pt(H(2)) , if E^(c-).(D(2)|...
Text Solution
|
- A solution containing H^(+) and D^(+) ions is in equilibrium with a mi...
Text Solution
|
- E(H^(+)//H(2))^(0)=0.00V, then E(D^(+)//D(2))^(0) at 25^(@)C will be
Text Solution
|
- If at same temperature and pressure, the densities for two diatomic ga...
Text Solution
|
- A solution containing H^(+) and D^(+) ions is in equilibrium with a mi...
Text Solution
|
- Find the inverse of each of the matrices given below : Let D= "dia...
Text Solution
|
- समान ताप तथा दाब पर दो द्वी -परमाणविक गैसों के घनत्वों के मान d(1) तथा...
Text Solution
|