A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
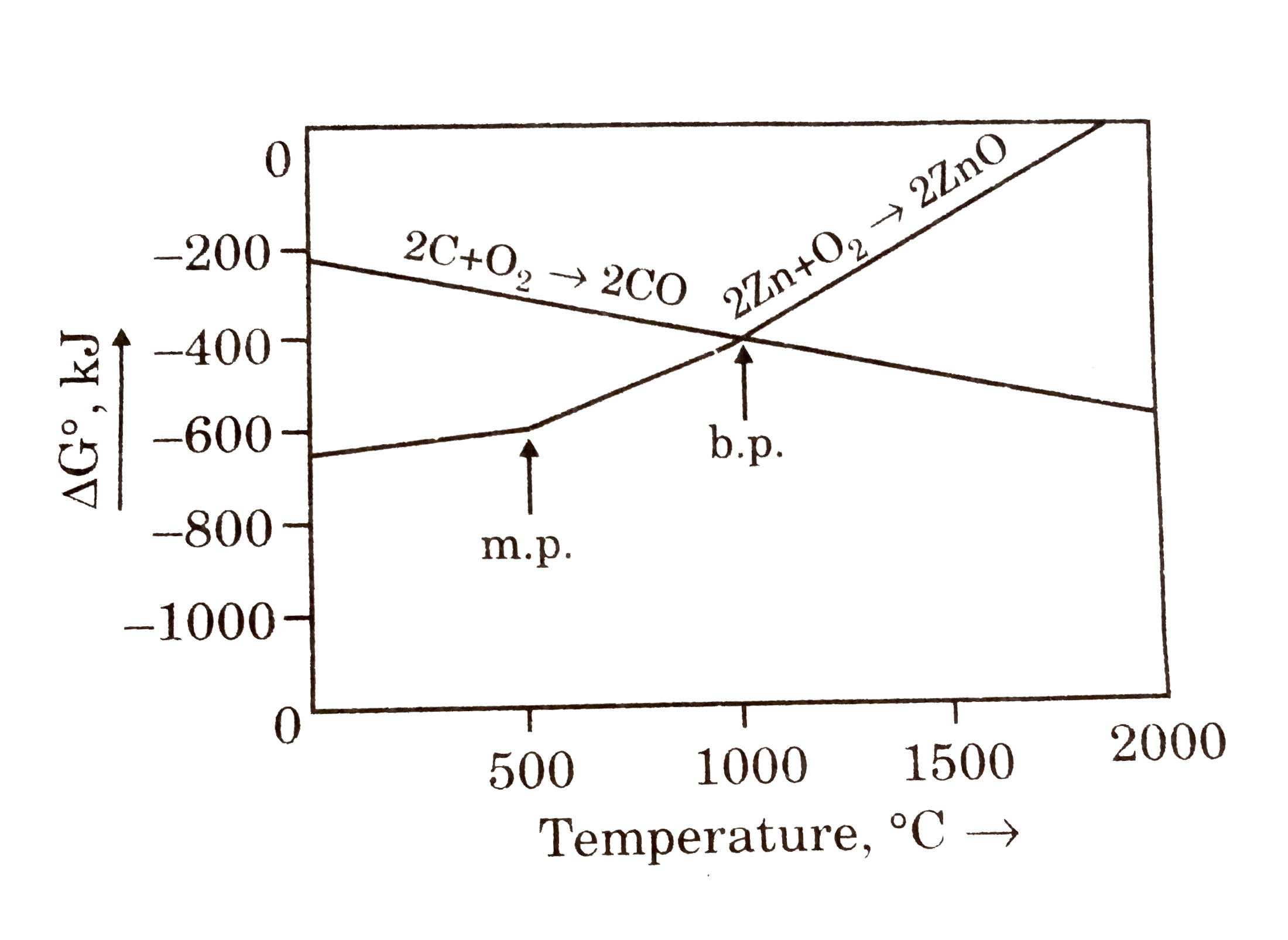
- Question given below are based on the given diagram of extractive meta...
Text Solution
|
- Questions given below are based on the given diagram for extractive me...
Text Solution
|
- Questions given below are based on the given diagram for extractive me...
Text Solution
|
- Questions given below are based on the given diagram for extractive me...
Text Solution
|
- Questions given below are based on the given diagram for extractive me...
Text Solution
|
- The points noted by arrows are melting and boiling points of the metal...
Text Solution
|
- Question given below are based on the given diagram of extractive meta...
Text Solution
|
- The points noted by arrows are melting and boiling points of the metal...
Text Solution
|
- Question given below are based on the given diagram of extractive meta...
Text Solution
|