A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
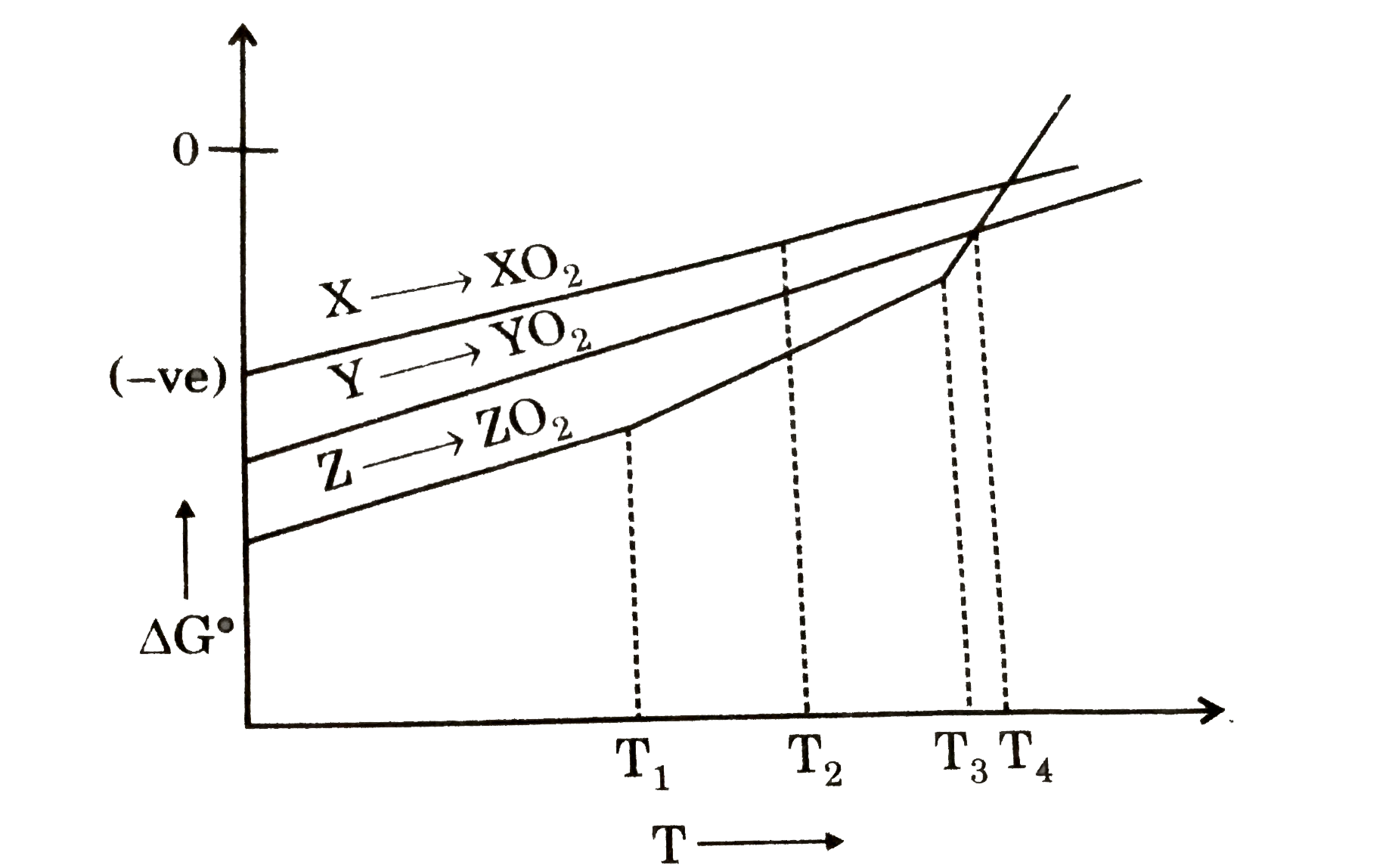
- AtT(4) temperature, the DeltaG for the reactioin Y+ZO(2) to Z+YO(2) is...
Text Solution
|
- Prove the identities: |[z, x, y],[ z^2,x^2,y^2],[z^4,x^4,y^4]|=|[x,...
Text Solution
|
- The values of x, y and z in the reaction are respectively: 4KO(2)+H(2)...
Text Solution
|
- Letvec(V) =v(x)hat(i)+v(y)hat(j)+v(z)hat(k), then int(t(1))^(t(2)) V(y...
Text Solution
|
- Z vs P is plotted for 1 mole of three different gases X,Y and Z at tem...
Text Solution
|
- AtT(4) temperature, the DeltaG for the reactioin Y+ZO(2) to Z+YO(2) is...
Text Solution
|
- An element Z forms an oxide ZO(3) . (a) What is the valency of element...
Text Solution
|
- दो पिण्ड विभिन्न तापों T(1) व T(2) पर हैं । यदि उन्हें उष्मीय संपर्क ...
Text Solution
|
- यदि sin^(-1)x+sin^(-1)y+sin^(-1)z=pi सिद्ध कीजिए कि x^(4)+y^(4)+z^(4...
Text Solution
|