The ortho-effect : This is special effect that is shown by o-substituents of bezene and its derivaties, but not necessarily just a steric, effect, e.g, the basicities of some o-substituted anilines were explanined in terms of steric effect and difference in crowding. round the nitrogen atom. This ortho-effectn alos operates with the benzen acids. Irrepective of the polar type, nearlly all o-substituted benzoic acids,are stronger than benzoic acid. As we have seen, benzoic acid is a resonance hybrid, and so the carboxyl groups is coplnar wiht the ring, An o-substituent tends to provent this coplanarity, Thus resonance is diminished (or prevented ), and so the O-atom of the OH groups has a greater positive charge, resulting increased acid strength. It follows from this tahat the greater the stric inhibition to resonane, the stronger is the rod . Support for this is the followings. order of strengths of substitued beznoic acids.
`2,6-di-Me(pK_(a)3.21)gt2-t-Bu(pK_(a) 3.46)gt2-Me(pK_(a) 3.91)`
Here again, if we consider the stability of the anion, stric, inhibition of resonacne prevents the +R effect of the ring coming into oprations (see above) and since this weakens acid strength its absence results in incresed acid strngth.
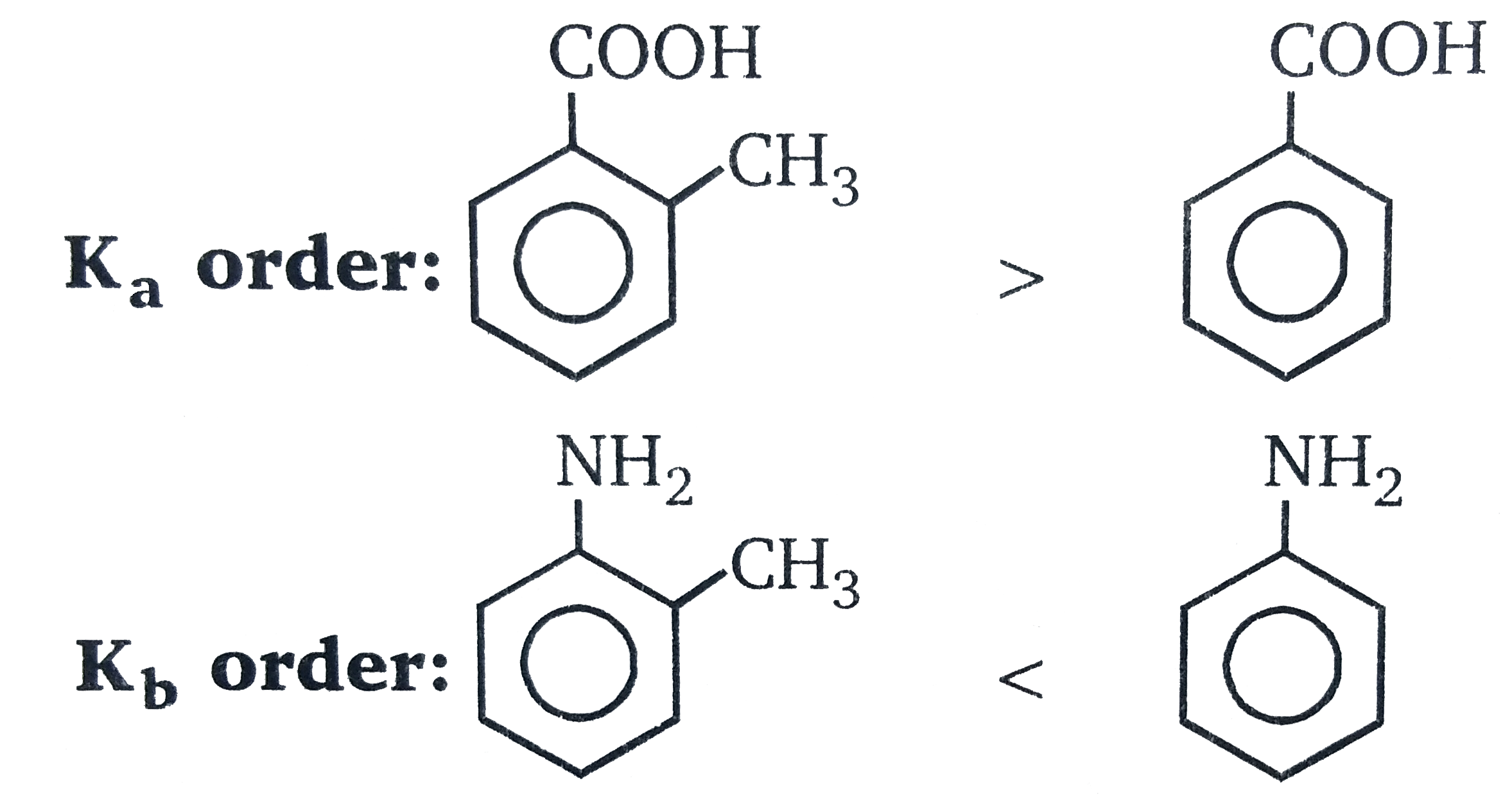
For options B and D, no ortho effect is valid and order of acidity and basicity is calculate by nearly examining the inductive effect.