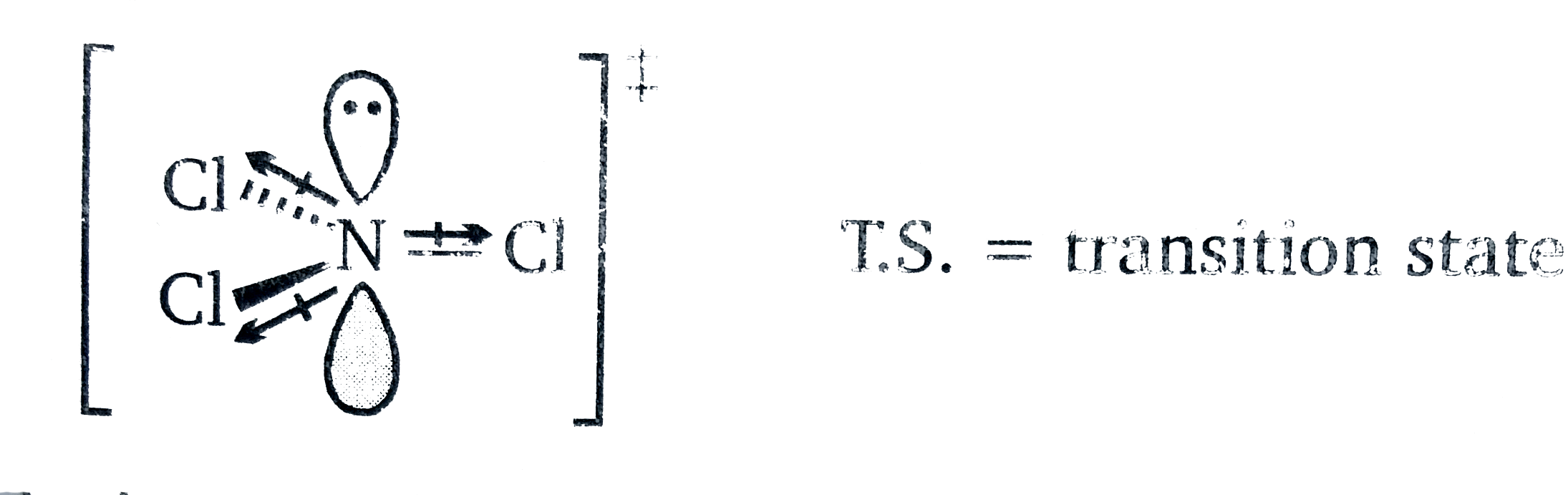(a) The barrier to inversion for `i-Pr_(2)` NMe is less than for `Me_(3)N` because going from `sp^(3)` (tetrahcedral ) to `sp^(2)` (trigonal planar ) sperads the bulky isopropyl groups futher apart and relieves steric crowing . In other words the isopropyl groups destabilize the pyramidal amine more than the planar transition structure.
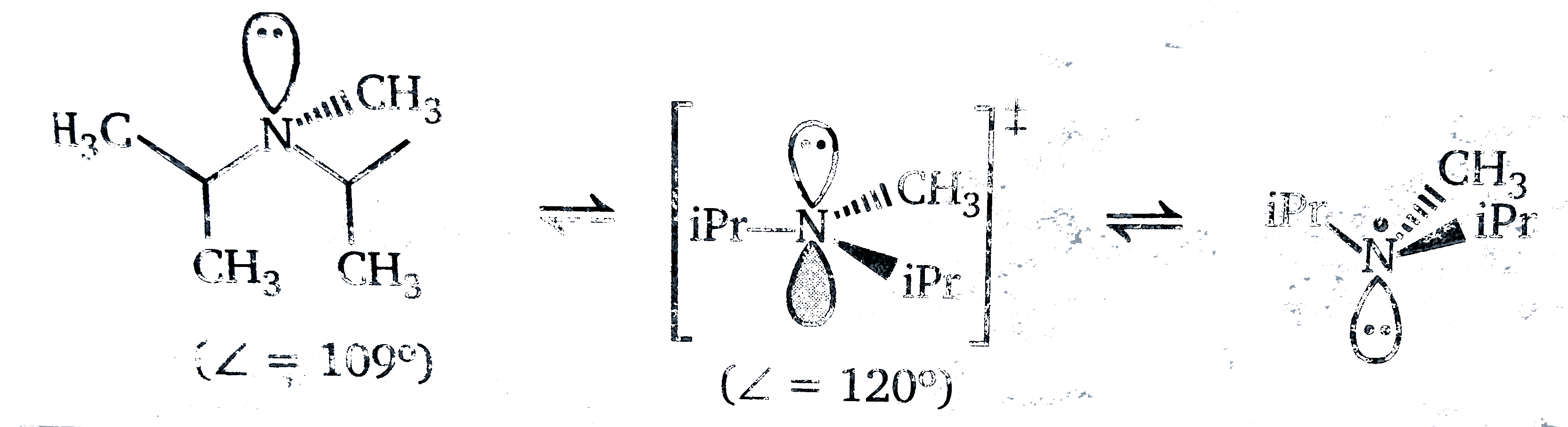
(b) The `sp^(2)` transition state has ideal bond angle of `~120^(@)` . The member rings T.S is highly strained becaouse it cannot achieve `120^(@)` due to the small ring locking the anges at `~60^(@)` . The-5 membered ring can easily adopt `120^(@)` angle .
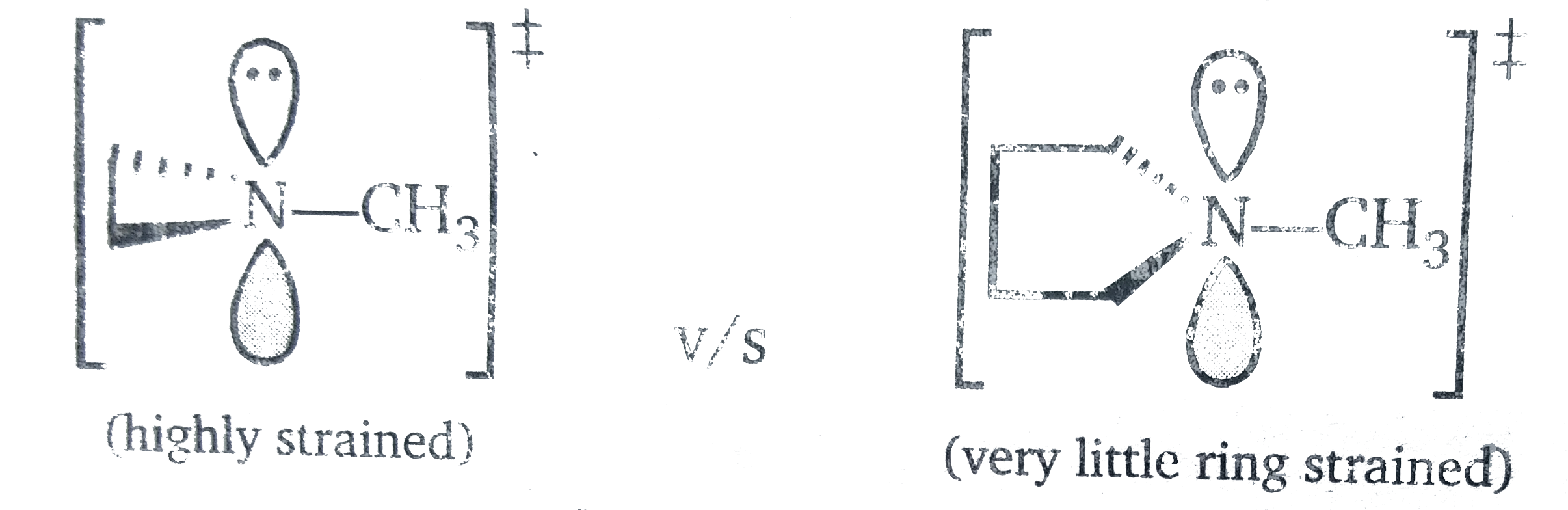
(c) As the nitrogen goes from `sp^(3)rarrsp^(2)` , It becomes effectively more electronegative. (Remember : more s-character means more electronegative ) The electronegative chlorine atoms pull electron density away from N more than the methyl groups, creating an electron deficient N, and destabilising the T.S