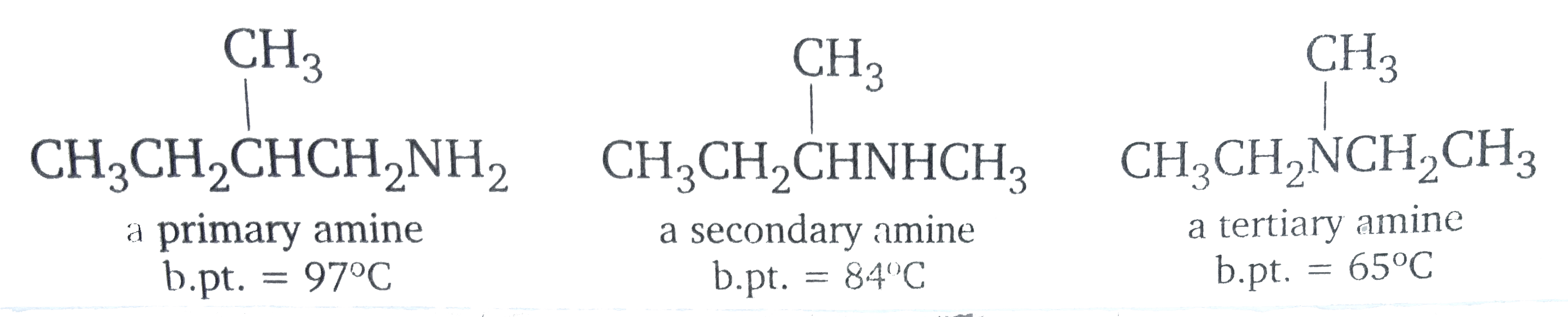Primary and secondary amines alos from hydrogen bonds, so these amines have higher boiling points than alkanes with similar molecular weights. Nitrogen is not as electronegative as oxygen , however, which , means that the hydrogen bonds between amine moelcules are weaker than the hydrogen alcohol molecules. Amines, therefore , have lower boiling points than alcohols with similar molecular weights.
Beacuse primary amines have two N-H bonds, hydrogen bonding is more significant for primary amines than for secondary amines. Tertiary amines amines cannot from hydrogen bonds with each other because they do not have a hydrogen attached to the bitrogen. Consequently, if you compare amines with the same molecular weight and similar structures, primary amines have higher boiling points than secondary amines, and secondary amines have higher boiling points than tertiary amines.