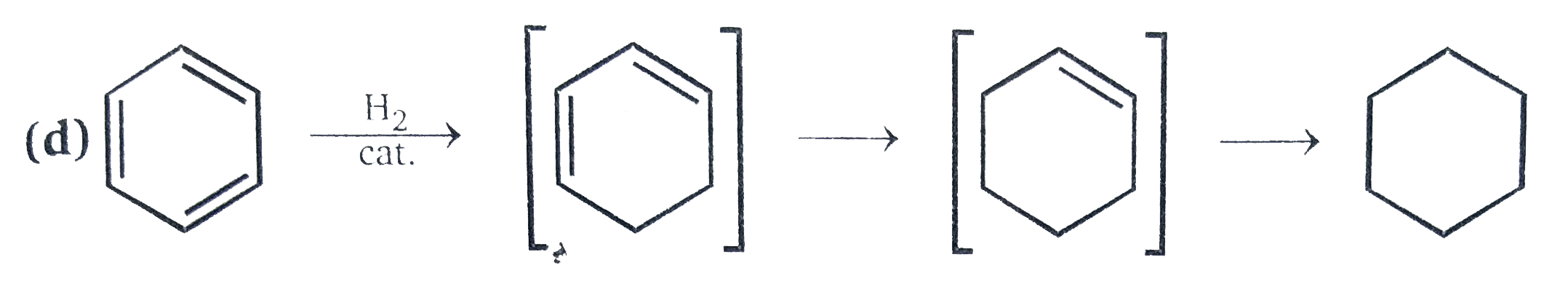
Addtion of one molecules of hydrogen produced cyclohexadiene, in which almost all of the resonacne energy of benzene has been lost. Hence the activation ,energy of this step is much greater than thart required for each succeeding step in which he double bonds behave like their acyclic analogues. Thus we have multi-step reaction in which the first step is retedermining . This means that the conditions required for the first step are more vigourus than those requred for the successive steps (all steps involve the addition of one molecule of hydrogen ) . Because of this, it is not possible to stop the reaction proceeding to comlete reduction of benzene to cyclohexane, and consequently it is not possible to isolate the intermediates.