A
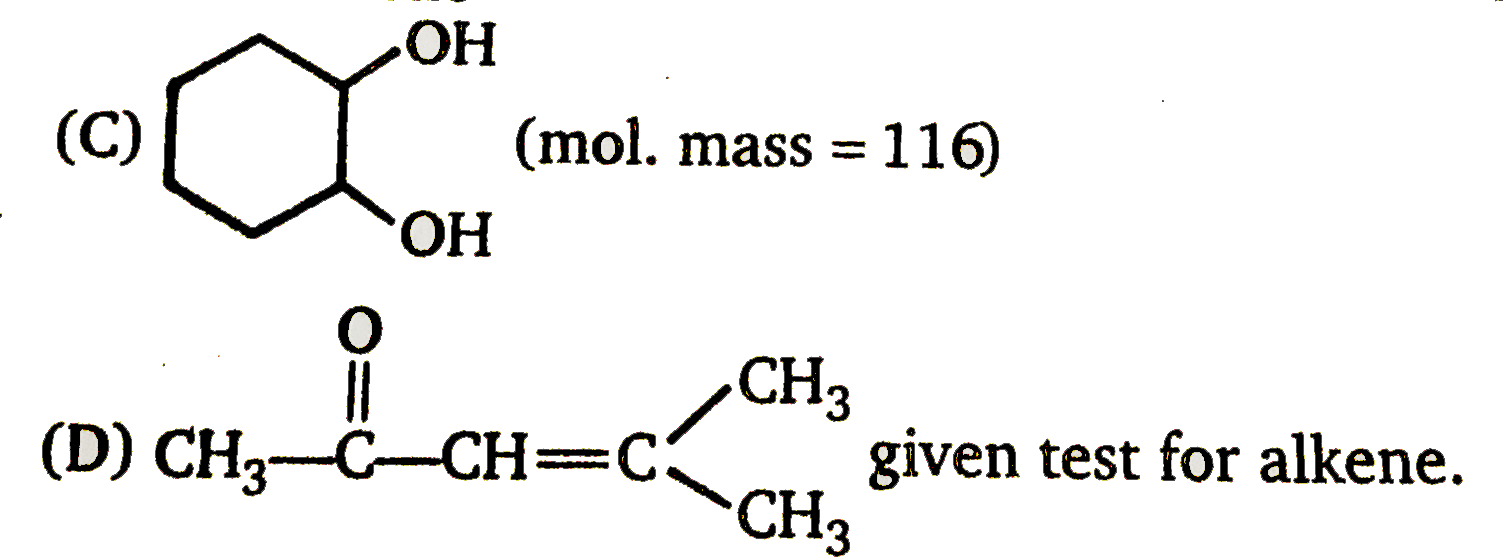
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDOL AND CANNIZARO REACTION
MS CHOUHAN|Exercise Level 1 (Q.1 To Q.30)|6 VideosALDOL AND CANNIZARO REACTION
MS CHOUHAN|Exercise Level 1 (Q.31 To Q.48)|3 VideosALDEHYDES AND KETONES II. ALDOL REACTIONS
MS CHOUHAN|Exercise ADDITIONAL OBJECTIVE QUESTIONS (LINKED COMPREHENSION TYPE )|6 VideosALKENES AND ALKYNES II
MS CHOUHAN|Exercise ADDITIONAL OBJECTIVE QUESTIONS (Matrix Match Type)|2 Videos
Similar Questions
Explore conceptually related problems
MS CHOUHAN-ALDOL AND CANNIZARO REACTION-Subjective Problems
- During an experimental workup procedure, a chemist treated a starting ...
Text Solution
|
- X=number of compound obtained by aldol reaction Y=Number of compound...
Text Solution
|
- x=moles of HCHO consumed. Value of (x) will be.
Text Solution
|
- CH(3)-overset(O)overset(||)(C)-CH(3)+CH(3)-CH(2)-overset(O)overset(||)...
Text Solution
|
 .
.