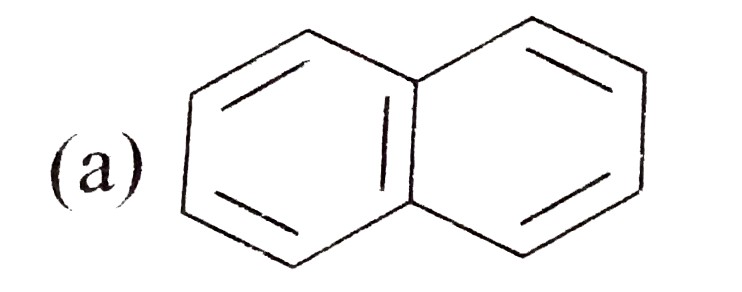
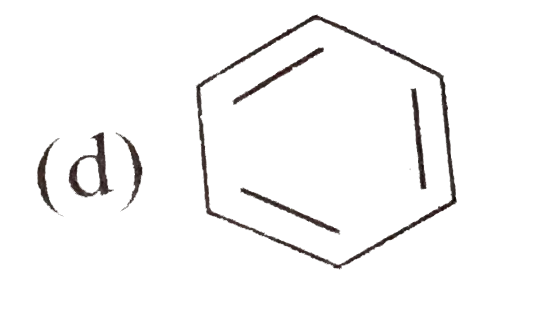
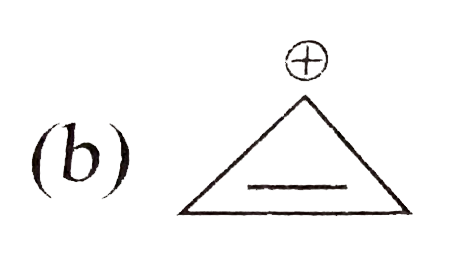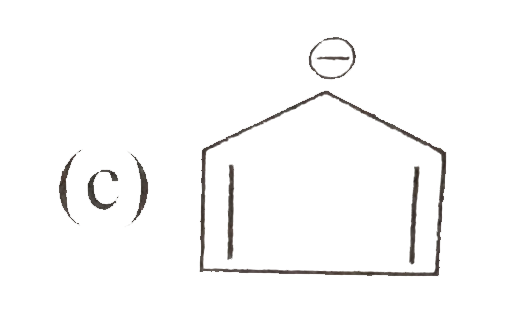A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.176 To Q.200)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.201 To Q.205)|5 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.126 To Q.150)|25 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-GENERAL ORGANIC CHEMISTRY-Level 2 (Q.151 To Q.175)
- Which one of the following compounds is not aromatic?
Text Solution
|
- Which one of the following compounds is the strongest acid?
Text Solution
|
- Which of following pairs are tautomers?
Text Solution
|
- The keto isomer of the following compound is :
Text Solution
|
- Which of the following compounds will exhibit tautomerism?
Text Solution
|
- Arrange the following compounds in increasing order of their heat of c...
Text Solution
|
- Arrange the following in increasing order of their heat of hydrogenati...
Text Solution
|
- Arrange the following in increasing order of their heat of combustion ...
Text Solution
|
- Arrange the following in increasing order of heat of hydrogenation :
Text Solution
|
- Arrange the following in decreasing order of heat of hydrogenation :
Text Solution
|
- The correct acidic strength order of acidic hydrogen P,Q and R is resp...
Text Solution
|
- The correct basicity order of atoms X,Y and Z is :
Text Solution
|
- Which of the following compounds do not have all C-C bond of same leng...
Text Solution
|
- Find out correct order of energy required for heterolytic cleavage of...
Text Solution
|
- Find out correct order for the energy requried for heterolytic cleavag...
Text Solution
|
- Find out correct representation of singlet carbene :
Text Solution
|
- Find out correct representation of triplet carbene :
Text Solution
|
- Which of the following orders is correct order of these anions?
Text Solution
|
- The barrier for rotation about indicated bonds will be maximum in whic...
Text Solution
|
- The most stable canonical structure of this molecule is :
Text Solution
|