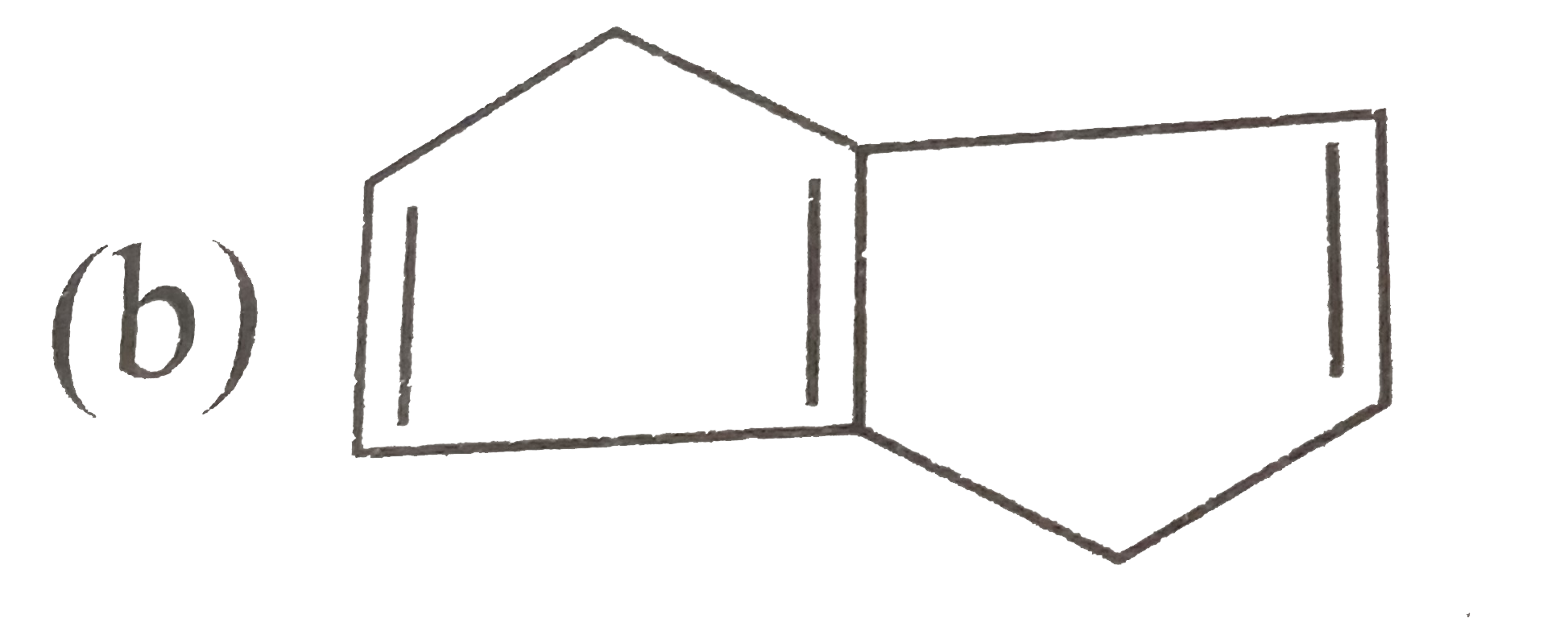
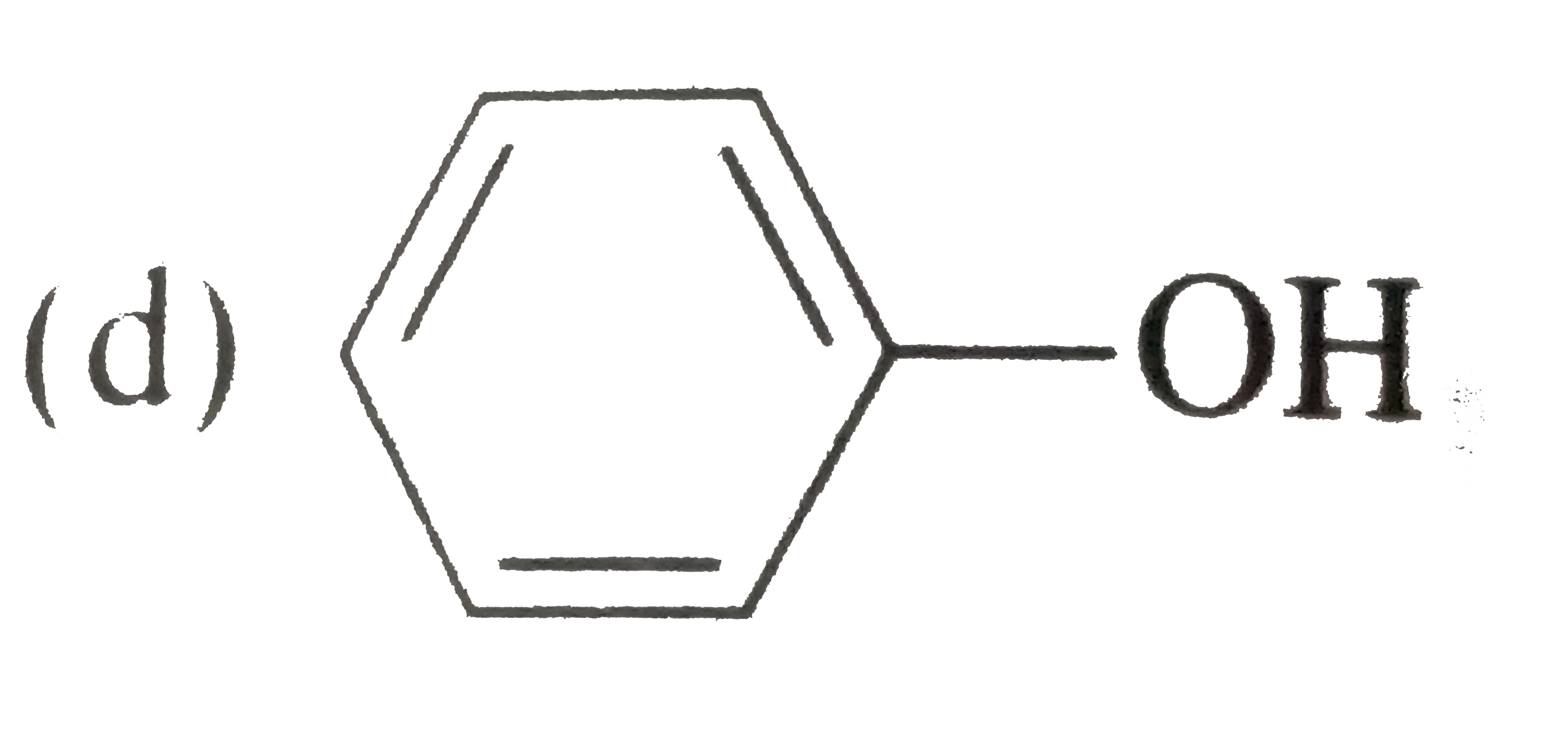
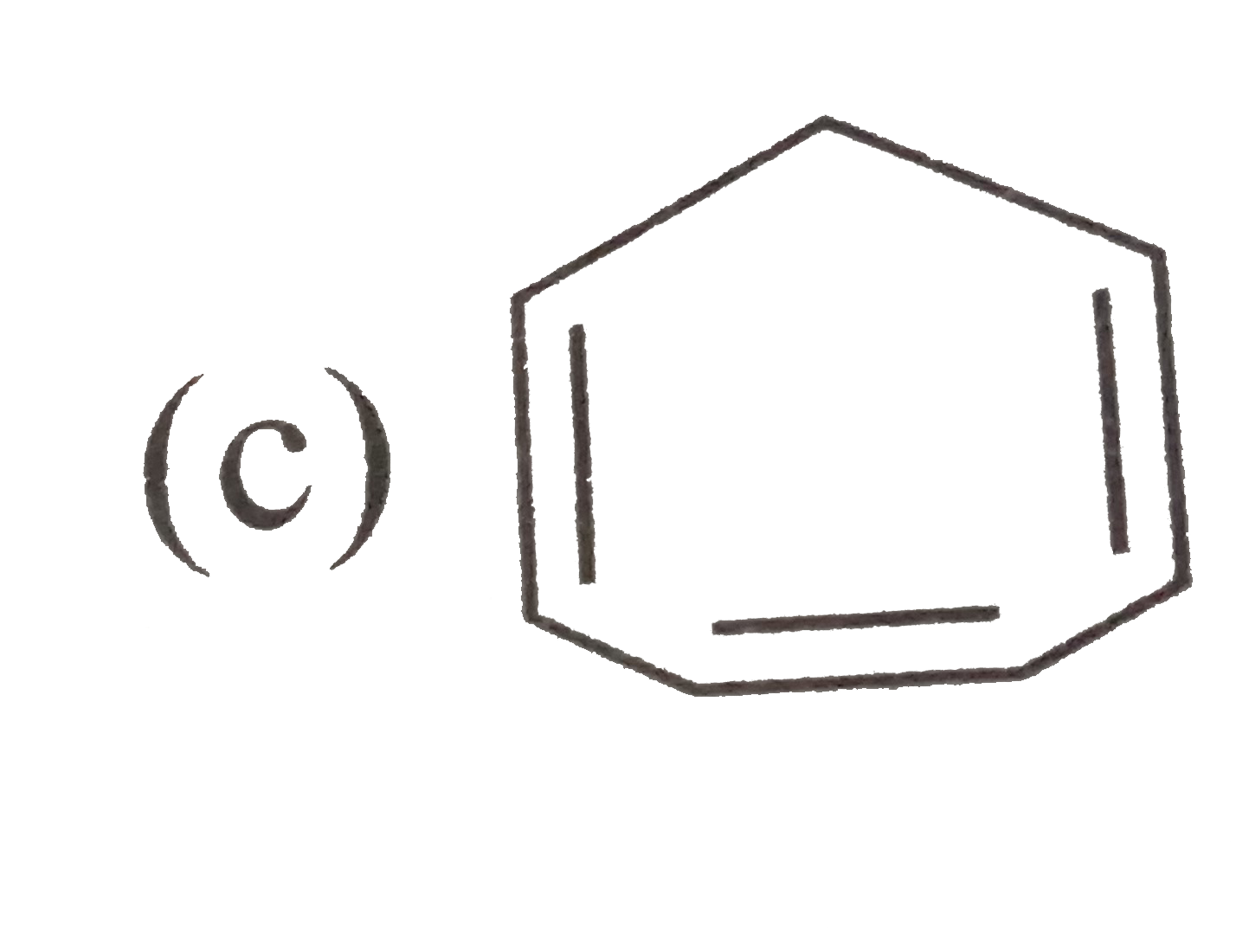A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise More Than One Correct (Q.1 To Q.25)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise More Than One Correct (Q.26 To Q.50)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.176 To Q.200)|25 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-GENERAL ORGANIC CHEMISTRY-Level 2 (Q.201 To Q.205)
- Find out anti aromatic compound among the following :
Text Solution
|
- Identify the compounds in which all bond length are equal :
Text Solution
|
- Which of the following alkene has highest value of heat of hydrogenati...
Text Solution
|
- Which of the following compound will not liberate CO(2) on reaction wi...
Text Solution
|
- Which of the following will not react with Na metal?
Text Solution
|