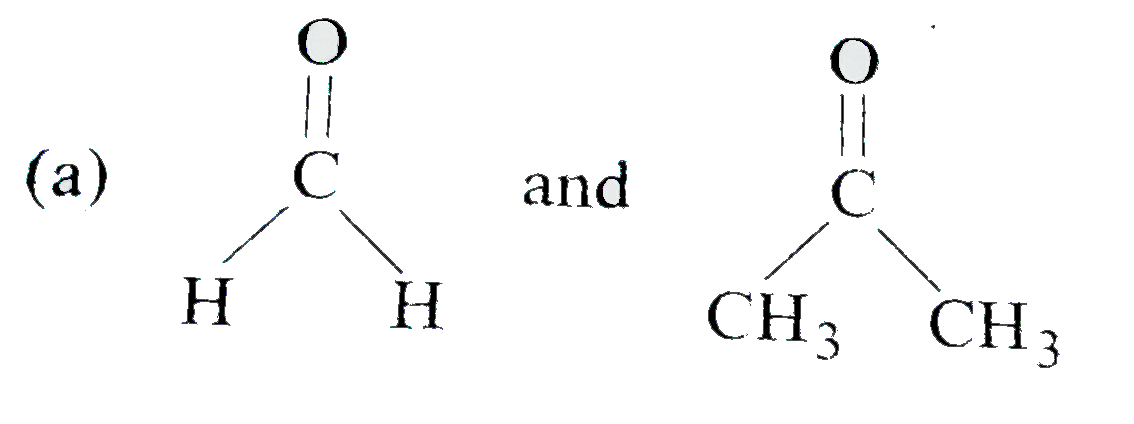
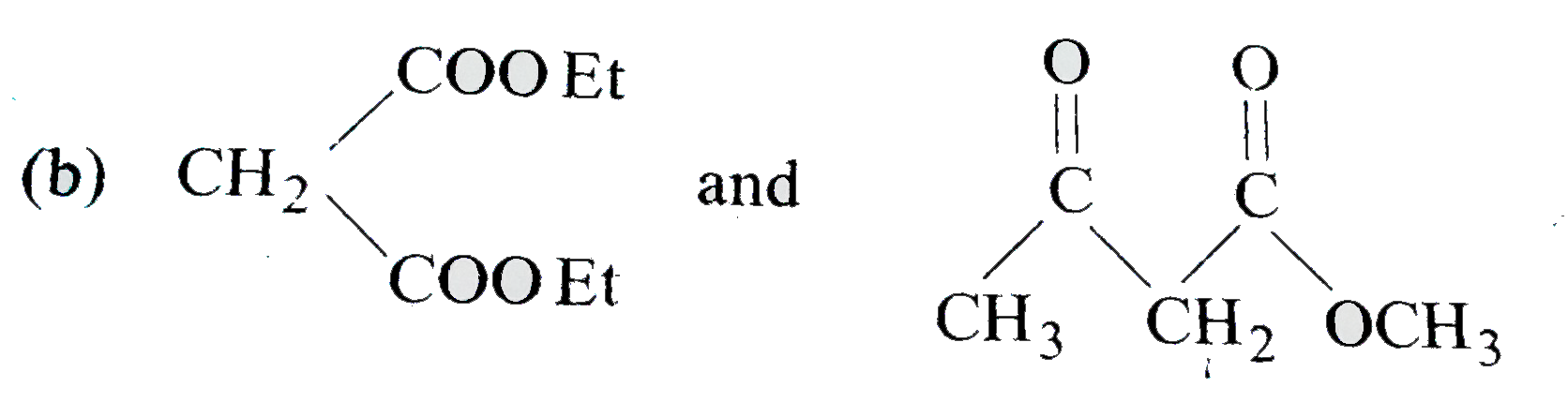
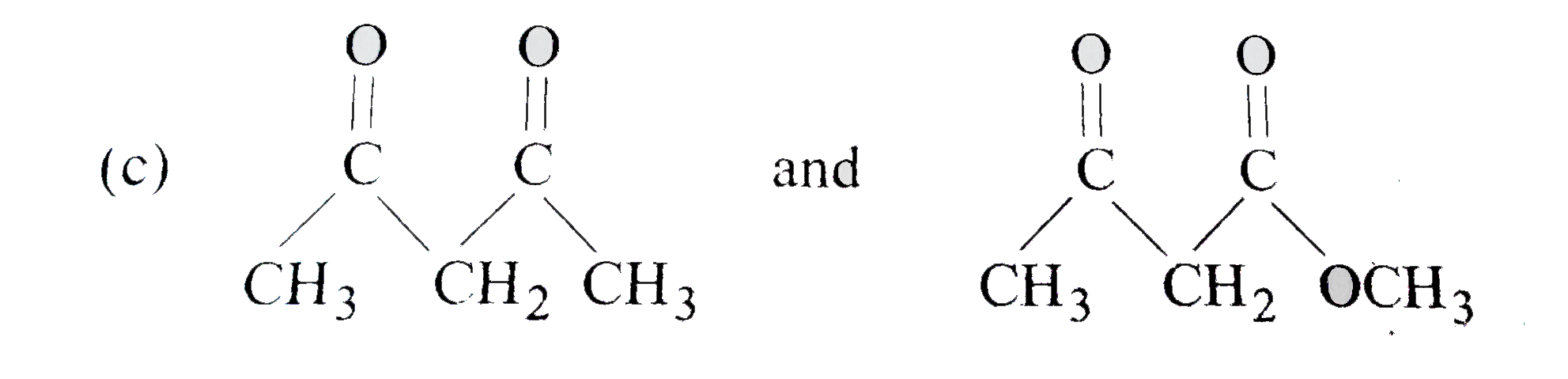
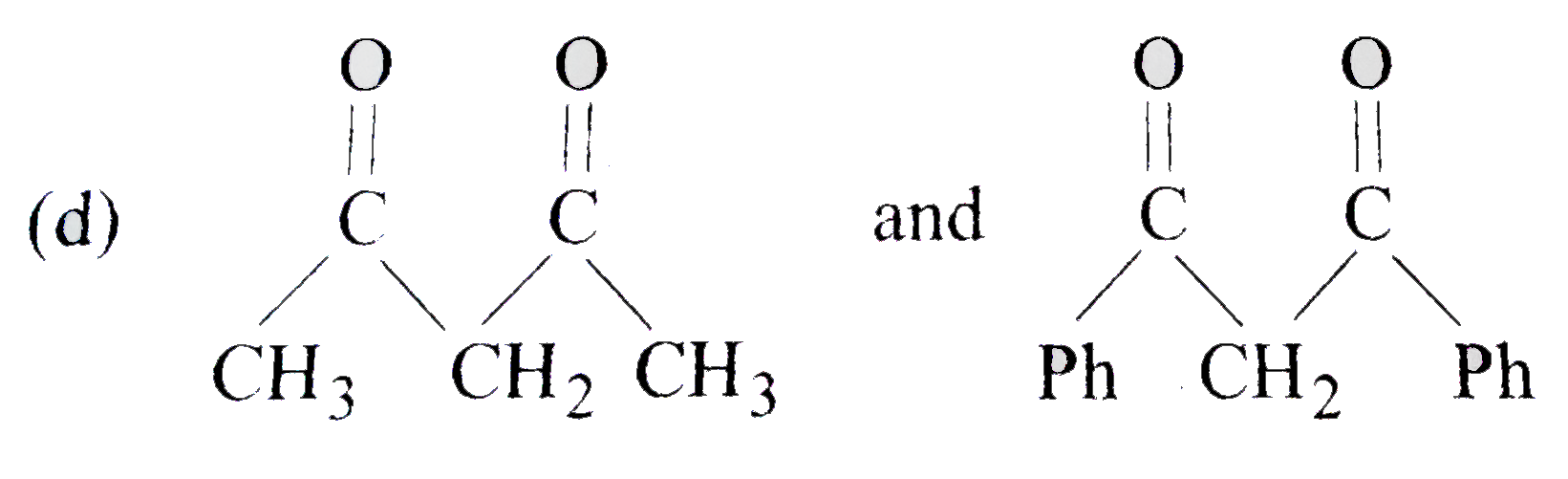
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS
HIMANSHU PANDEY|Exercise Level 1 (Q.51 To Q.1)|2 VideosCARBONYL COMPOUNDS
HIMANSHU PANDEY|Exercise Level 2 (Q.1 To Q.25)|24 VideosCARBONYL COMPOUNDS
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 VideosCABOXYLIC ACIDS AND ITS DERIVATIVES
HIMANSHU PANDEY|Exercise Subjective Type Problems|8 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-CARBONYL COMPOUNDS-Level 1 (Q.26 To Q.50)
- In the given reaction C(6)H(5)CHO+X underset((ii)HOH//NH(4)Cl)overs...
Text Solution
|
- Cannizzaro reaction is example of:
Text Solution
|
- Cross Cannizzaro reaction is an example of :
Text Solution
|
- Which will be give silver mirror test with Tollen's reagent?
Text Solution
|
- Acetaldehyde cannot give:
Text Solution
|
- The reaction in which KCN//C(2)H(5)OH is used is :
Text Solution
|
- Which one of the following reactions is used for the conversion of ket...
Text Solution
|
- Schiff's reagent gives pink colour with :
Text Solution
|
- Consider the sturcture of given alcohol, this alcohol can be prepared ...
Text Solution
|
- In the given reaction [X] will be : C(6)H(5)-underset(O)underset(||)...
Text Solution
|
- In the reaction sequence, [X] is which keton ? [X]overset(KMnO(4)//o...
Text Solution
|
- In the given reaction, [X] will be :
Text Solution
|
- What is the given reaction known as ? CH(3)-overset(O)overset(||)C-C...
Text Solution
|
- In the given reaction (X) and (Y) will respectively be : X+Y underse...
Text Solution
|
- Which of the following compounds are in their most stable tautomeric f...
Text Solution
|
- Which of the following compounds have higher enolic content than keto ...
Text Solution
|
- In which of the following pairs , the first one will have a higher eno...
Text Solution
|
- Most stable Tautomer of the following compound is :
Text Solution
|
- underset((ii)C(6)H(5)Ona)overset((i)Br(2),H^(+))rarr P,P will be
Text Solution
|
- Which of the following is an example of aldol condensation ?
Text Solution
|