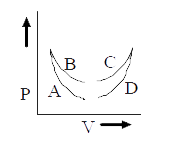A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HEAT-2
MOTION|Exercise EXERCISE-2 (LEVEL-I)|24 VideosHEAT-2
MOTION|Exercise EXERCISE-2 (LEVEL-II)|13 VideosHEAT-2
MOTION|Exercise EXERCISE-4 (LEVEL-II)|30 VideosHEAT TRANSFER & THERMAL EXPANSION
MOTION|Exercise Exercise - 3 Section-B|19 VideosHYDROSTATIC, FLUID MECHANICS & VISCOSITY
MOTION|Exercise EXERCISE -3 (SECTION-B) PREVIOUS YEAR PROBLEM|7 Videos
Similar Questions
Explore conceptually related problems
MOTION-HEAT-2 -EXERCISE-1
- An ideal system can be brought from stage A to B through four paths as...
Text Solution
|
- A system is given 400 calories of heat and 1000 Joule of work is done ...
Text Solution
|
- For a thermodynamic process delta Q=- 50 calorie and W =-20 calorie. I...
Text Solution
|
- One mole of an ideal gas at temperature T(1) expends according to the ...
Text Solution
|
- One mole of an ideal diatomic gas is taken through the cycle as shown ...
Text Solution
|
- When a system is taken from state 'a' to state 'b' along the path 'acd...
Text Solution
|
- In the above question, if the work done on the system along the curved...
Text Solution
|
- In above question, if U(a) = 40J, value of U(b) will be
Text Solution
|
- A diatomic gas of moleculer weight 30 gm/mole is filled in a container...
Text Solution
|
- An ideal gas undergoes the process 1 to 2 as shown in the figure, the ...
Text Solution
|
- If heat is added at constant volume, 6300 J of heat are required to ra...
Text Solution
|
- A reversible adiabatic path on a P-V diagram for an ideal gas passes t...
Text Solution
|
- Four curves A, B, C and D are drawn in Fig. for a given amount of gas....
Text Solution
|
- In reference of above figure, no heat exchange between the gas and the...
Text Solution
|
- During the adiabatic change of ideal gas, the realation between the pr...
Text Solution
|
- The pressure of the gas filled in thermally insulated container is P a...
Text Solution
|
- A thermodynamics cycle takes in heat energy at a high temperature and ...
Text Solution
|
- A cylindrical tube of cross-sectional area A has two air tight frictio...
Text Solution
|
- A carnot engine works between ice point and steam point. It is desired...
Text Solution
|
- A cylindrical tube of uniform cross-sectional area A is fitted with tw...
Text Solution
|