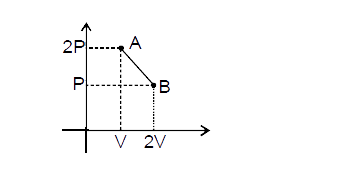
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HEAT-2
MOTION|Exercise EXERCISE-2 (LEVEL-II)|13 VideosHEAT-2
MOTION|Exercise EXERCISE-3 (LEVEL-I)|22 VideosHEAT-2
MOTION|Exercise EXERCISE-1|40 VideosHEAT TRANSFER & THERMAL EXPANSION
MOTION|Exercise Exercise - 3 Section-B|19 VideosHYDROSTATIC, FLUID MECHANICS & VISCOSITY
MOTION|Exercise EXERCISE -3 (SECTION-B) PREVIOUS YEAR PROBLEM|7 Videos
Similar Questions
Explore conceptually related problems
MOTION-HEAT-2 -EXERCISE-2 (LEVEL-I)
- An ideal gas undergoes an adiabatic process obeying the relation PV^(4...
Text Solution
|
- One mole of an ideal gas at STP is heated in an insulated closed conta...
Text Solution
|
- O(2) is 16 times heavier that H (2). If at same temperatue the O(2) mo...
Text Solution
|
- A flask is filled with 13 g of an ideal gas at 27^(@)C and its tempera...
Text Solution
|
- Which of the following quantities is the same for all ideal gases at t...
Text Solution
|
- According to kinetic theory of gases,
Text Solution
|
- One mole of an ideal monoatomic gas at temperature T(0) expands slowel...
Text Solution
|
- In case of hydrogen and oxygen at N.T.P., which of he following quanti...
Text Solution
|
- A closed container is fully insulated from outside. One half of it is ...
Text Solution
|
- The differential form of first law of thermodynamics is
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure the fraction...
Text Solution
|
- The process AB is shown in the diagram. As the gas is taken from A to ...
Text Solution
|
- One mole of a gas expands obeying the relation as shown in the P/V dia...
Text Solution
|
- An ideal gas expands from volume V (1) to V (2). This may be achieved ...
Text Solution
|
- What is/are the same for O (2) and NH(3) in gaseous state
Text Solution
|
- In the following P-V diagram two adiabatics cut two isothermals at tem...
Text Solution
|
- Two curves are given at temperatures T(1) and T(2) in an isothermal pr...
Text Solution
|
- Three curves are shown in the P-V diagram. P, Q and R represent the pr...
Text Solution
|
- A vertical cylinder with heat-conducting walls is closed at the bottom...
Text Solution
|
- When heat is supplied to the gas it expands and displaces piston by L/...
Text Solution
|