A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-HEAT-2 -EXERCISE-4 (LEVEL-II)
- A cylinder of mass 1kg is given heat of 20,000J at atmospheric pressur...
Text Solution
|
- Match the following for the given process :
Text Solution
|
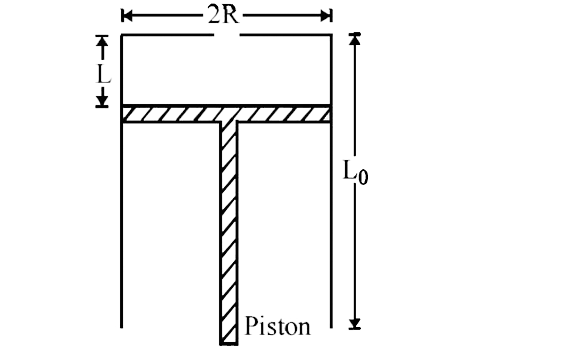
- A fixed thermally conducting cylinder has a radius R and height L0. Th...
Text Solution
|
- A fixed thermally conducting cylinder has a radius R and height L0. Th...
Text Solution
|
- A fixed thermally conducting cylinder has a radius R and height L0. Th...
Text Solution
|
- Statement-1: The total translational kinetic energy of fall the molecu...
Text Solution
|
- An ideal gas is expanding such that PT^(2)= constant. The coefficient ...
Text Solution
|
- Column I contains a list of processes involving expansion of an ideal ...
Text Solution
|
- Cv and Cp denote the molar specific heat capacities of a gas at consta...
Text Solution
|
- The figure shows the P-V plot of an ideal gas taken through a cycle AB...
Text Solution
|
- A real gas behaves like an ideal gas if its
Text Solution
|
- One mole of an ideal gas in initial state A undergoes a cyclic process...
Text Solution
|
- A diatomic ideal gas is compressed adiabatically to 1/32 of its initia...
Text Solution
|
- 5.6 liter of helium gas at STP is adiabatically compressed to 0.7 lite...
Text Solution
|
- One mole of a monatomic ideal gas is taken through a cycle ABCDA as s...
Text Solution
|
- A mixture of 2 moles of helium gas ((atomic mass)=4a.m.u) and 1 mole o...
Text Solution
|
- Two moles of ideal helium gas are in a rubber balloon at 30^@C. The ba...
Text Solution
|
- Two non-reactive monoatomic ideal gases have their atomic masses in th...
Text Solution
|
- One mole of a monatomic ideal gas is taken along two cyclic processes ...
Text Solution
|
- In Fig., a container is shown to have a movable (without friction) pis...
Text Solution
|