A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MODERN PHYSICS - 2
MOTION|Exercise EXERCISE - 4 (Level -II) PREVIOUS YEAR - JEE ADVANCED|28 VideosMODERN PHYSICS - 2
MOTION|Exercise EXERCISE - 3 (Level -II) SUBJECTIVE - JEE ADVANCED (SECTION D)|2 VideosMODERN PHYSICS
MOTION|Exercise PHYSICS|73 VideosMODERN PHYSICS -1
MOTION|Exercise EXERCISE-4 ( LEVEL- II)|39 Videos
Similar Questions
Explore conceptually related problems
MOTION-MODERN PHYSICS - 2-EXERCISE - 4 (Level -I) PREVIOUS YEAR - JEE MAIN
- The energy spectrum of beta - particle [number N€ as a function of bet...
Text Solution
|
- If the binding energy per nucleon in .(3)^(7)Li and .(2)^(4)He nuclei ...
Text Solution
|
- An alpha nucleus of energy (1)/(2)m nu^(2) bombards a heavy nucleus o...
Text Solution
|
- Which of the following transitions in a hydrogen atom emits photon of ...
Text Solution
|
- In gamma ray emission from a nucleus
Text Solution
|
- If M(O) is the mass of an oxygen isotope .(8)O^(17),M(p) and M(n) are ...
Text Solution
|
- The half-life period of a radio-active element X is same as the mean l...
Text Solution
|
- Assertion: Energy is released when heavy nuclei undergo fission or lig...
Text Solution
|
- The alongside is a plot of binding energy per nucleon E(b) ,against th...
Text Solution
|
- A radioactive nucleus (initial mass number A and atomic number Z) emit...
Text Solution
|
- A nucleus of mass M + Deltam is at rest and decays into two daughter n...
Text Solution
|
- A nucleus of mass M + Deltam is at rest and decays into two daughter n...
Text Solution
|
- The half-life of a radioactive substance is 20 min. The approximate ti...
Text Solution
|
- Statement-1:A nucleus having energy E(1) decays by beta^(-) emission t...
Text Solution
|
- Assume that a neutron breaks into a proton and an electron. The energy...
Text Solution
|
- If a simple pendulum has significant amplitude (up to a factor of1//e ...
Text Solution
|
- Half-lives of two radioactive elements A and B are 20 minutes and 40 m...
Text Solution
|
- A radioactive nucleus A with a half life T, decays into a nucleus B. A...
Text Solution
|
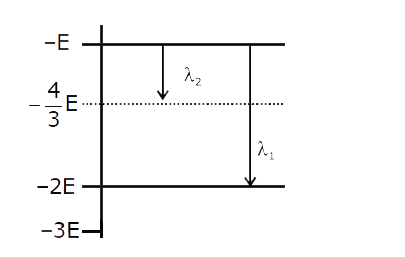
- Some energy levels of a molecule are shown in the figure. The ratio of...
Text Solution
|
- It is found that if a neutron suffers an elastic collinear collision w...
Text Solution
|